Method for synthesizing 1, 8-cineole by catalyzing alpha-terpilenol through solvent-free system
A terpineol, solvent-free technology, applied in the field of organic synthesis, can solve the problems of low selectivity and no practical value of 1,8-cineole, and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The method for catalyzing α-terpineol to synthesize 1,8-cineole in a solvent-free system comprises the following steps:
[0018] Step 1: Add 0.364g of cetyltrimethylammonium bromide and 2.880g of phosphotungstic acid to 30 mL of ethanol with a volume fraction of 95% in sequence, reflux and stir at 90°C for 10 hours, and the stirring speed is 900 rpm. The solvent was removed under reduced pressure, and vacuum-dried at 60°C for 3 hours to obtain 3.100 g of white solid catalyst hexadecyltrimethylammonium phosphotungstic acid;
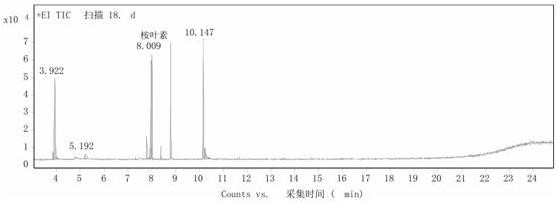
[0019] Step 2: Add 1.000g cetyltrimethylammonium phosphotungstic acid to 10mL α-terpineol, heat to 60°C and stir for 10 hours at a stirring speed of 900 rpm. After the reaction, cool the reaction product to 5 ℃, the catalyst was recovered by suction filtration, and the obtained oily product was detected by GC ( figure 1 ), with toluene as the internal standard, the calculated 1,8-cineole content was 40%, and the residual α-terpineol content was 5%....
Embodiment 2
[0021] The method for catalyzing α-terpineol to synthesize 1,8-cineole in a solvent-free system comprises the following steps:
[0022] Step 1: Add 0.640g of hexadecyltrimethylammonium chloride and 5.760g of phosphotungstic acid to 60 mL of ethanol with a volume fraction of 95% in sequence, reflux and stir at 90°C for 10 hours, and the stirring speed is 900 rpm. The solvent was removed under reduced pressure, and vacuum-dried at 60°C for 6 hours to obtain 6.188 g of white solid catalyst hexadecyltrimethylammonium phosphotungstic acid;
[0023] Step 2: Add 0.120g of cetyltrimethylammonium phosphotungstic acid into 2mL of α-terpineol, heat to 55°C and stir for 10 hours at a stirring speed of 900 rpm. After the reaction, cool the reaction solution to 5 ℃, the catalyst was recovered by suction filtration, and the obtained oily product was detected by GC ( figure 2 ), using toluene as the internal standard, the calculated 1,8-cineole content was 33%, and the residual α-terpineol ...
Embodiment 3
[0025] The method for catalyzing α-terpineol to synthesize 1,8-cineole in a solvent-free system comprises the following steps:
[0026] Step 1: Add 0.207g of n-octyltrimethylammonium chloride and 1.440g of phosphotungstic acid to 20 mL of ethanol with a volume fraction of 95% in sequence, and reflux and stir at 90°C for 8 hours at a stirring speed of 900 rpm. The solvent was removed under pressure, and vacuum-dried at 60°C for 4 hours to obtain 1.600 g of white solid catalyst n-octyltrimethylammonium phosphotungstic acid;
[0027] Step 2: Add 0.240g of n-octyltrimethylammonium phosphotungstic acid into 8mL of α-terpineol, heat to 60°C and stir for 10 hours at a stirring speed of 900 rpm. After the reaction, cool the reaction solution to 5°C , the catalyst was recovered by suction filtration, and the obtained oily product was detected by GC ( image 3 ), using toluene as the internal standard, the calculated 1,8-cineole content was 35%, and the residual α-terpineol content was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com