Modification of xylanase to improve thermophilicity, alkophilicity and thermostability
A technology of xylanase and polycanase, applied in the field of xylanase, can solve the problem of not developing thermophilic and basophilic family 11 xylanase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Construction of Trichoderma Reesei modified xylanase NI-TX
[0139] Basic recombinant DNA methods such as plasmid preparation, restriction enzyme digestion, polymerase chain reaction, oligonucleotide phosphorylation, ligation, transformation, and DNA hybridization are based on well-established methods well known to those skilled in the art (Sung et al. ., 1986) or according to the method recommended by the manufacturer of the enzyme or kit. Buffers for many enzymes are supplied as part of a kit or reconstituted according to the manufacturer's recommendations. Restriction enzymes, T4 polynucleotide kinase and T4 DNA ligase were purchased from New England BioLabs LTD, Mississauga, Ont. GeneAmp PCR Kit was purchased from Perkin-Elmer. The starting plasmid pXYbc, a pUC-type plasmid inserted with the Bacillus circulans xylanase gene, had been prepared and published before (Sung et al, 1993; Campbell et al, US Patent 5,405,769, April 11, 1995 date authorized). A common E....
Embodiment 2
[0305] Construction of mutant xylanase NI-BX from Bacillus circulans
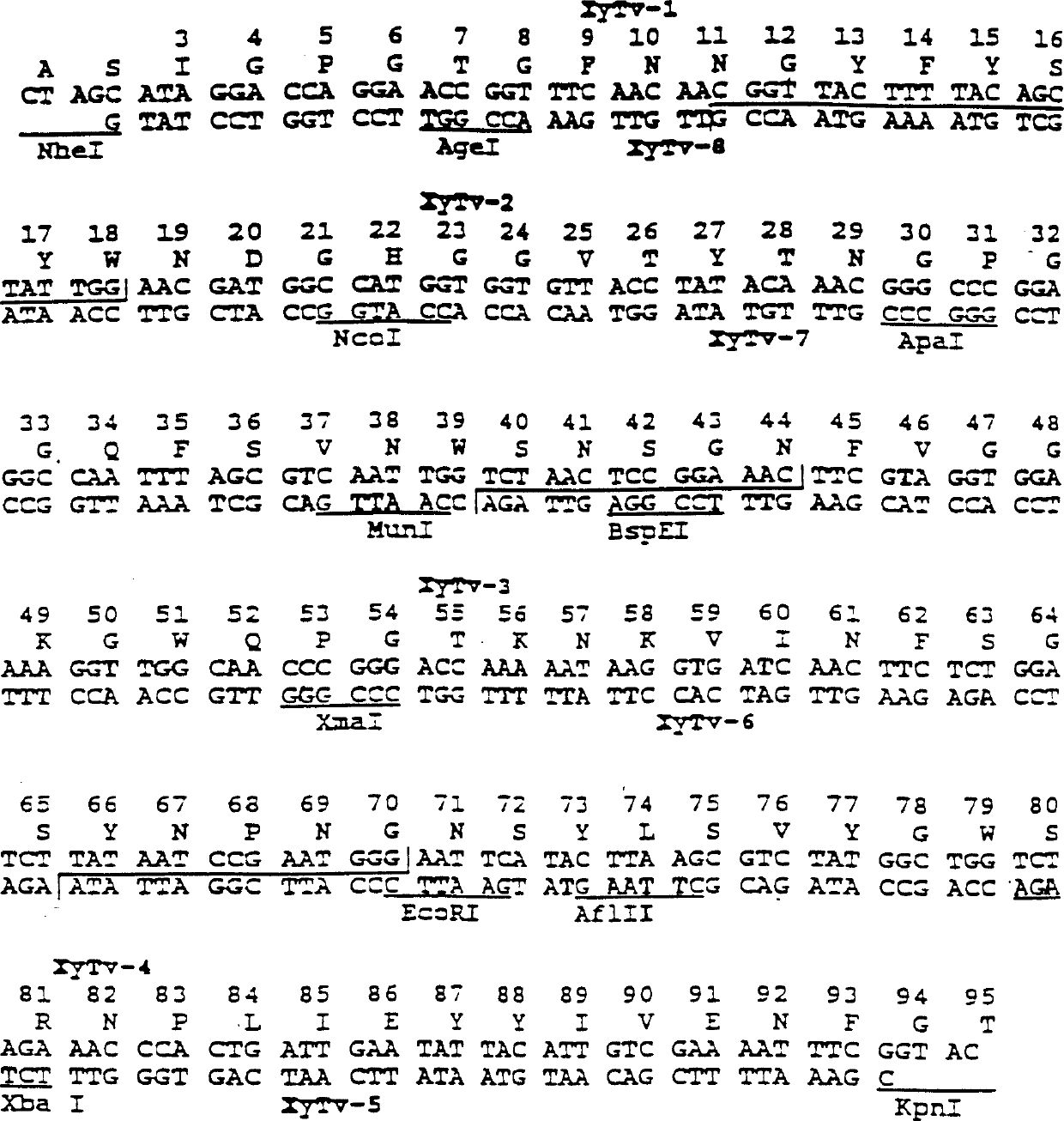
[0306]Modifications NI-TX2, NI-TX3, NI-TX7, NI-TX8 and NI-TX9 of the fungal Trichoderma xylanase are also reproduced in the bacterial Bacillus circulans xylanase (BcX). The starting plasmid pXYbc has been prepared and published before (Sung et al, 1993; Campbell et al, US Patent 5,405,769, issued April 11, 1995), and is only briefly described here. Following the same procedure described above for pXyTv(3-190), assemble the coding circular by enzymatic phosphorylation with T4 DNA kinase and ligation of overlapping synthetic oligonucleotides into a linearized plasmid by T4 DNA ligase. Synthetic gene for Bacillus xylanase (BcX) (Figure 4) (Sung et al, 1993).
[0307] A. Construction of plasmid pNI-BX1
[0308] The modification method of NI-BX1 has been used in the preparation of NI-TX2. Mutant NI-BX1 is a modified version of BcX, which replaces the (1-22) region of BcX with the Tfx (1-31) sequence. The con...
Embodiment 3
[0370] Generation and analysis of modified xylanases
[0371] (A) Production of xylanase
[0372] The culture conditions were the same as well-established methods for the production of other E. coli expressed xylanases. 5 ml of an overnight culture in 2YT medium (16 g yeast extract, 10 g tryptone, 5 g NaCl, 1 L water) containing ampicillin (100 mg / L) was added to 2YT medium containing ampicillin (1 L). Shake culture at 37° C. (200 rpm), and harvest the cells after 16 hours.
[0373] (B) Purification of modified xylanase
[0374] To prepare protein samples from cells, 10 g of cell samples were first ground with 25 g of aluminum powder to prepare cell extracts. After grinding into a homogeneous mixture, add a small amount (5 mL) of ice-cold buffer A (10 mM sodium acetate, pH 5.5, for the BcX mutant) or buffer B (10 mM sodium acetate, pH 4.6, for the TX mutant) , grinding the mixture vigorously between each addition. The mixture was centrifuged at 8000xg for 30 minutes to re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com