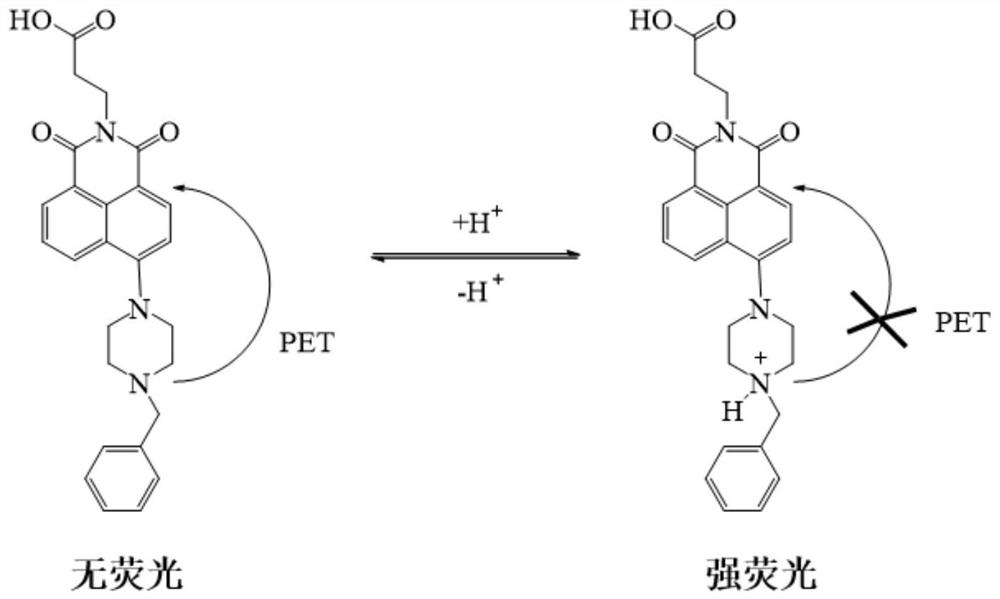
A kind of 1,8-naphthalimide derivative and its application
A technology of naphthalimide and derivatives, which is applied in the field of fluorescent probes, can solve problems affecting sensitivity, etc., and achieve high sensitivity and selectivity, good water solubility, and reduced interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
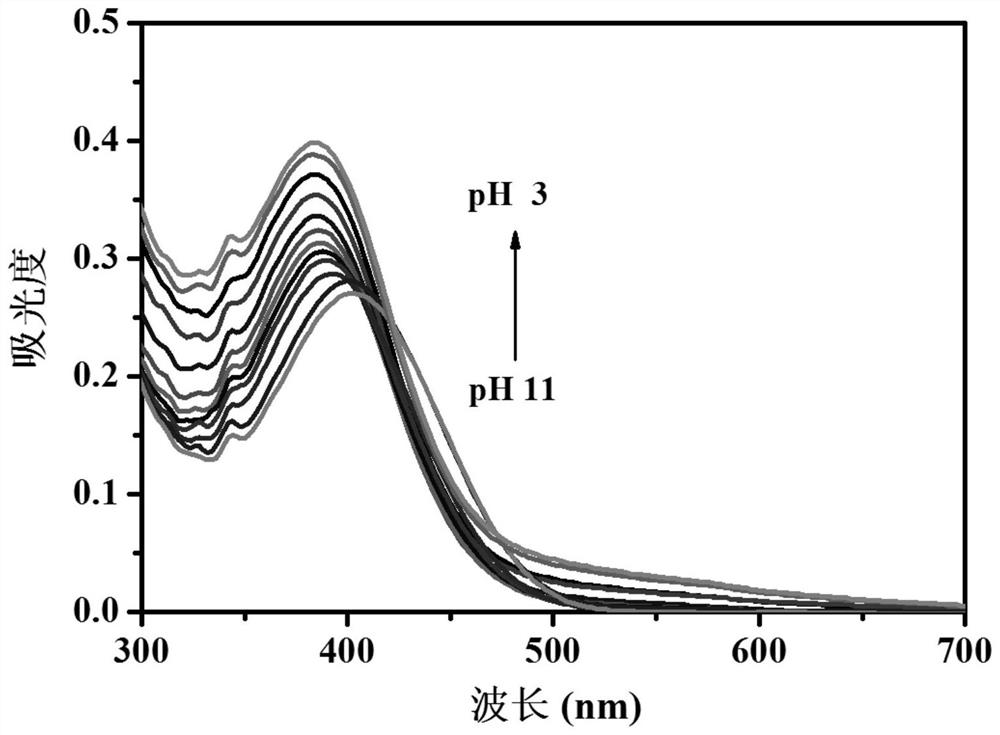
[0028] Probe 3-[6-(4-benzylpiperazin-1-yl)-1,3-dioxo-1H-benzo[iso]quinolin-2(3H)-yl] in Example 1 Propionic acid was stored in a stock solution prepared with a concentration of 1 mM in dimethyl sulfoxide. In the experiment, the probe was diluted to a final concentration of 10 μM with phosphate buffer with different pH values, and the UV absorption spectrum of the probe was recorded as the pH changed ( figure 2 ). As the pH value decreased from 11.00 to 3.00, the absorption peak at 402nm gradually increased and blue-shifted to 383nm. At the same time, the color of the solution changed from colorless to yellow ( image 3 ).
Embodiment 2
[0030] Similarly, the probe 3-[6-(4-benzylpiperazin-1-yl)-1,3-dioxo-1H-benzo[iso] in Example 1 was used in phosphate buffer with different pH values Quinoline-2(3H)-yl]propionic acid was diluted to a final concentration of 10 μM, the excitation wavelength was fixed at 402 nm, and the fluorescence emission spectrum changes of the probe with pH changes ( Figure 4 ). As the pH value decreased from 11.00 to 3.00, the fluorescence intensity at 526nm gradually increased. The Stokes shift is as high as 124nm, which is beneficial to reduce the interference of excitation light during imaging. Under the irradiation of ultraviolet light, the color of the solution changed from colorless to bright green ( Figure 5 ). pK was calculated from the Singmoidal fitting curve of the fluorescence intensity value at 526 nm as a function of pH a The value is 6.69 ( Image 6 ), the pH response linear range is 6.40-8.00 (the linear regression equation is I=-229144.02×pH+1.87×10 6 , the linear c...
Embodiment 3
[0032] Keep the probe 3-[6-(4-benzylpiperazin-1-yl)-1,3-dioxo-1H-benzo[iso]quinolin-2(3H)-yl]propanoic acid concentration at At 10 μM, respectively examine the probe in the presence of common metal ions, the H + selectivity. like Figure 8 As shown, when the pH is 4.0, 6.8, 7.4, the probe has almost no response to the above substances, which proves that the probe is sensitive to H + Has high selectivity. Figure 8 The sequence and concentration of the substances in it are: 1, Blank; 2, Mg 2+ (1mM); 3, Pd 2+ (1mM); 4, Ca 2+ (1mM); 5, K +(1mM); 6, Zn 2+ (1mM); 7, Fe 3+ (1mM); 8, Sr 2+ (1mM); 9, Ni 2+ (1mM); 10, Al 3+ (1mM); 11, Ba 2+ (1mM); 12, Mn 2+ (1mM); 13, Na + (1mM); 14. Histidine(1mM); 15. Glutamate(1mM); 16. Aspartic acid(1mM); 17. Leucine(1mM); 18. Phenylalanine(1mM); 19. Lysine(1mM); 20. Alanine 21, Serine (1mM); 22, Leucine (1mM); 23, Arginine (1mM); 24, Proline (1mM); 25, Threonine (1mM); 26, Tryptophan (1mM); 27, Isoleucine ( 1 mM); 28. Cysteine (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com