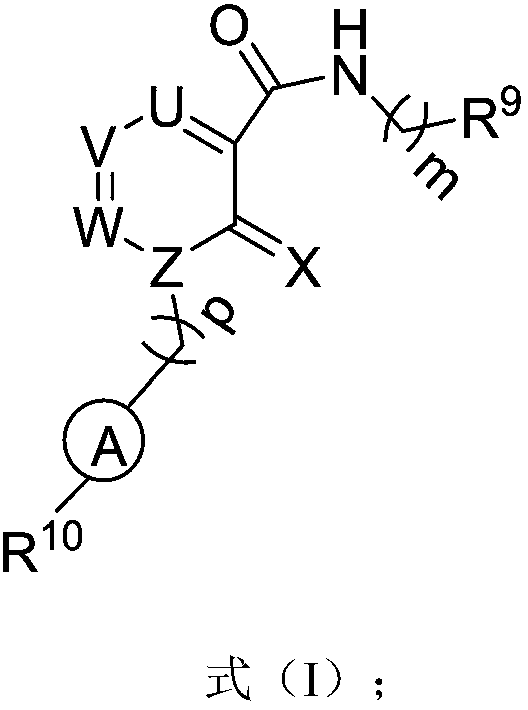
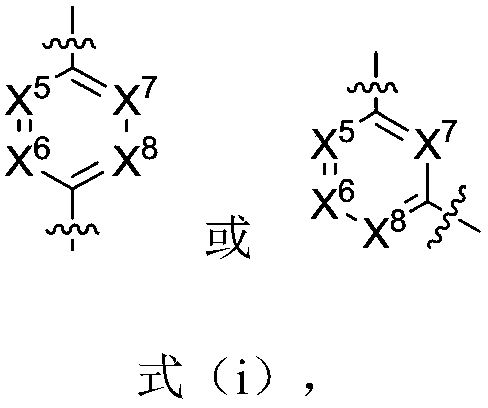
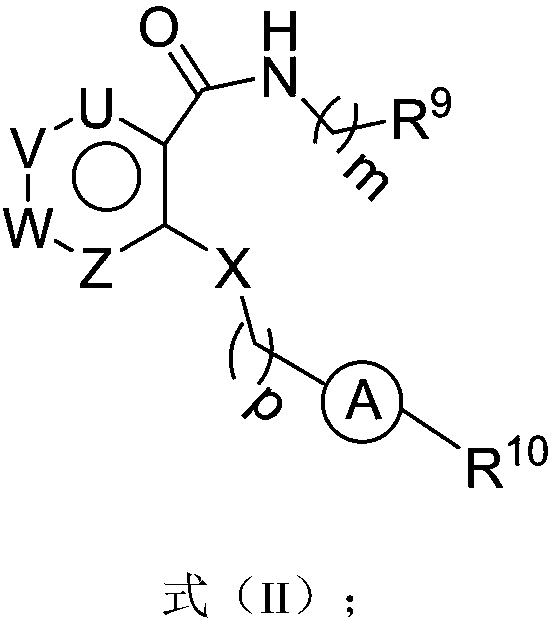
Six-membered ring formamide compounds, synthesis method and applications thereof
A technology for cyclic formamides and compounds, which is applied in the field of six-membered cyclic formamide compounds and their synthesis, and can solve problems that need further research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0110] Example 1-1, Preparation of the compound N-(3,4-methylenedioxyphenyl)-2(-(4-(hydroxycarbamoyl)benzyl)oxy)benzamide (QZ001)
[0111]
[0112] Dissolve salicylic acid (180mg, 1.3mmol, 1eq) and N,N'-carbonyldiimidazole (CDI, 211mg, 1.3mmol, 1eq) in DMF (5ml), react at 40°C for 30min, and then add 1,8 - Diazabicycloundec-7-ene (DBU, 396mg, 2.6mmol, 2eq) and tert-butanol (193mg, 2.6mmol, 2eq) were reacted at 60°C for 12h. After the reaction, the solvent was removed under reduced pressure, extracted with ethyl acetate and water, and passed through a silica gel column after conventional treatment to obtain the intermediate tert-butyl benzoate (214rrmg, 1.1mmol).
[0113] The intermediate tert-butyl benzoate (214mg, 1.1mmol, 1.0eq) was dissolved in anhydrous acetonitrile (50ml), and methyl 4-bromomethylbenzoate (298mg, 1.3mmol, 1.2eq) and Cs 2 CO 3 (912mg, 2.8mmol, 2.5eq), react at room temperature for 4h. After the reaction was finished, the solvent was removed under red...
Embodiment 1-10
[0121] Embodiment 1-10, compound 1-(4-(hydroxycarbamoyl) benzyl)-N-(4-hydroxyphenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide ( QZ010) preparation.
[0122]
[0123] Dissolve 2-hydroxynicotinic acid (181mg, 1.3mmol, 1eq) and N,N'-carbonyldiimidazole (CDI, 211mg, 1.3mmol, 1eq) in DMF (5ml), react at 40°C for 30min, then add 1,8-diazabicycloundec-7-ene (DBU, 396mg, 2.6mmol, 1eq) and tert-butanol (193mg, 2.6mmol, 2eq) were reacted at 60°C for 12h. After the reaction, the solvent was removed under reduced pressure, extracted with ethyl acetate and water, and passed through a silica gel column after conventional treatment to obtain the intermediate tert-butyl 2-oxo-1,2-dihydropyridine-3-carboxylate (215 mg, 1.1 mmol).
[0124] Take the intermediate 2-oxo-1,2-dihydropyridine-3-carboxylic acid tert-butyl ester (215mg, 1.1mmol, 1eq) and dissolve it in anhydrous acetone (50ml), add 4-bromomethylbenzoic acid methyl Ester (252mg, 1.1mmol, 1.0eq) and K 2 CO 3 (304mg, 2.2mmol, 2eq),...
Embodiment 2
[0134] Example 2. Detection of IDO1 inhibitory activity of some compounds of the present invention
[0135]The plasmid construction containing human IDO1 gene in the present invention, the amplification and expression in Escherichia coli, and extraction and purification are all carried out according to the method reported by Christopher etc. (Austin, C.J., et al. (2004). Protein Expr Purif37 (2): 392-398.). The compound synthesized by the present invention is detected according to the following method: add 50 mM potassium phosphate buffer (PH 6.5), 10 mM sodium ascorbate, 10 μM methylene blue, 100 μg / ml catalase, 200 μM L-tryptophan in a 96-well plate, and different concentrations of inhibitors. The concentrations of inhibitors were set at 3.2805, 10.935, 36.45, 121.5, 405, 1350, 4500, 15000, 50000 nM. After incubating at 37°C for 5 minutes, IDO1 enzyme was added and reacted at 37°C for 30 minutes. Afterwards, 30% (w / v) trichloroacetic acid was added to terminate the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com