Preparation method of teriflunomide
A technology of teriflunomide and acetamide, which is applied in the field of preparation of teriflunomide, can solve the problems of unfavorable industrial production, corrosion of equipment, and low total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
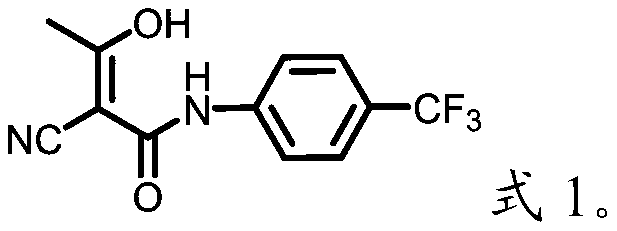
[0033] The invention provides a kind of preparation method of teriflunomide, comprises the following steps:
[0034] (1) Mix cyanoacetic acid, condensing agent, aprotic solvent and alkaline reagent, carry out condensation reaction, obtain active ester system;
[0035] (2) Mixing the active ester system with 4-trifluoromethylaniline for condensation reaction to obtain intermediate 2-cyano-N-(4-trifluoromethyl-phenyl)-acetamide;
[0036] (3) Mix the intermediate 2-cyano-N-(4-trifluoromethyl-phenyl)-acetamide with acetyl chloride for acylation reaction to obtain teriflunomide.
[0037] The invention mixes cyanoacetic acid, a condensing agent, an aprotic solvent and an alkaline reagent for condensation reaction to obtain an active ester system. In the present invention, the condensing agent is preferably CDI, Pybop, HATU, DCC, EDC.HCl, HOAt, HOBt, HCTU, TBTU or isobutyl chloroformate; the aprotic solvent is preferably acetone, acetonitrile, di Chloromethane, chloroform, carbon t...
Embodiment 1
[0052] (1) Dissolve 50g (0.59mol) cyanoacetic acid in 500mL dichloromethane and place in an ice bath; add 245g HATU (0.65mol) to the above reaction system and add 114.4g (1.6mol) N,N-diiso Propylethylamine was stirred in an ice bath for 1 hour to complete the condensation reaction, and the active ester system was obtained, which was directly used in the next step without purification and post-treatment.
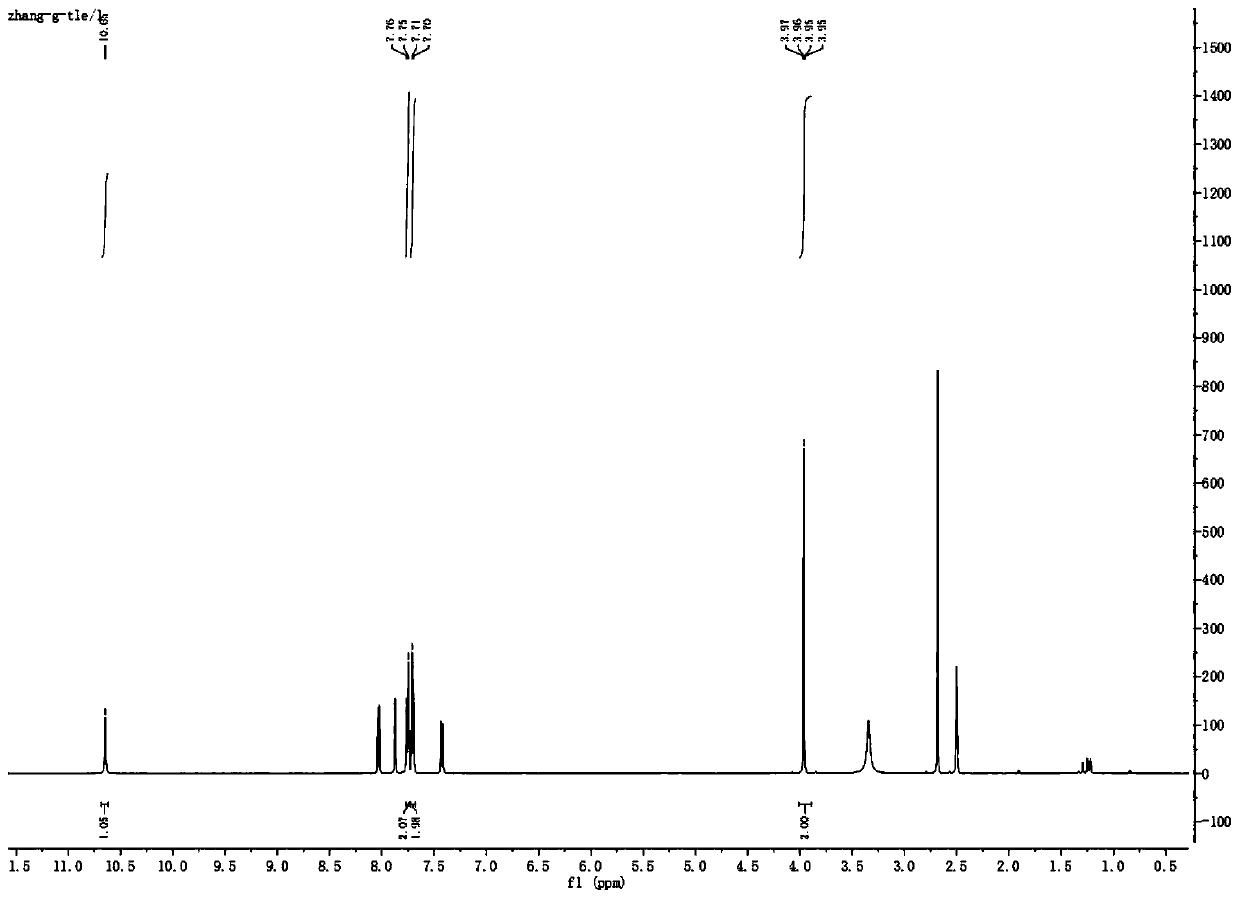
[0053] (2) Add 100 g (0.62 mol) of 4-trifluoromethylaniline into the active ester system, stir at 0° C. for 3 h to carry out condensation reaction, and follow the reaction by TLC until the reaction of the raw materials is complete. After the reaction, the reaction solution was poured into 1 L of ice water and stirred for 30 min, a large amount of light yellow solid was precipitated, filtered, the filter cake was washed with 500 mL of water, dried and weighed to obtain 110 g of the solid, with a yield of 82%.
[0054] The obtained light yellow solid compound is identified by p...
Embodiment 2
[0059] (1) Dissolve 50g (0.59mol) of cyanoacetic acid in 500mL of dichloromethane and place in an ice bath; add 368.4g (0.65mol) of PyBop into the above reaction system and add 161.9g (1.6mol) of triethylamine, ice After stirring in the bath for 1 h to complete the condensation reaction, the active ester system is obtained. This step does not require purification and post-treatment, and is directly used in the next reaction.
[0060] (2) With embodiment 1.
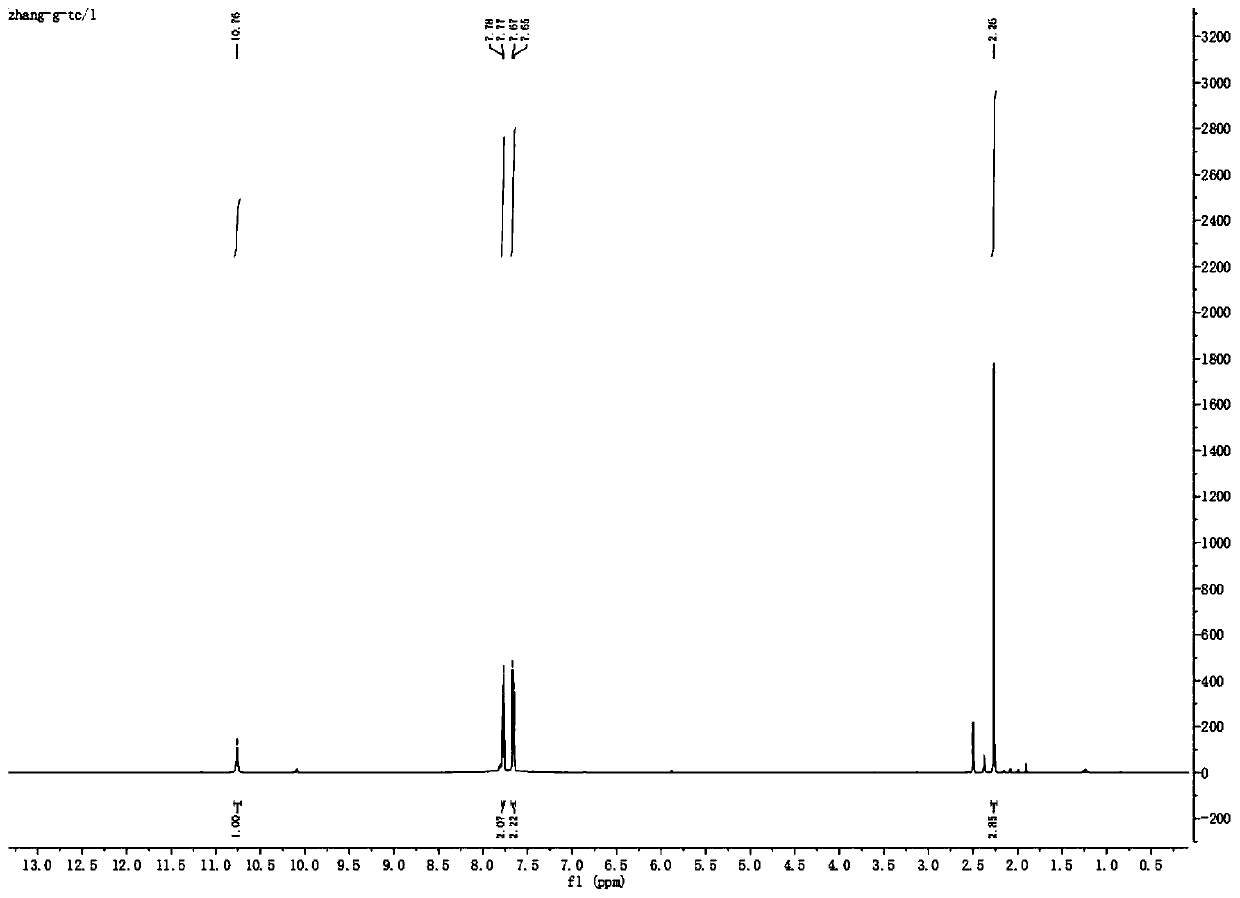
[0061] (3) 100g (0.44mol) of the intermediate 2-cyano-N-(4-trifluoromethyl-phenyl)-acetamide was dissolved in 1L of acetonitrile and placed in an ice bath, and 31.68g (1.32mol) was added for hydrogenation The sodium was reacted for 10 minutes, and then 41.5 g (0.53 mol) of acetyl chloride was added dropwise. After the drop was completed, the ice bath was removed, and the acylation reaction was carried out at a room temperature of 25° C., followed by TLC until the reaction of the raw materials was completed. After the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com