Purification method of beta-glucuronidase
A technology of glucuronidase and purification method, which is applied in the field of in vitro diagnosis, can solve the problems of low recovery rate, slow enzyme production, too many steps, etc., and achieve simple purification method, high development and application value, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 The purification method of β-glucuronidase of the present invention comprises the following steps:
[0019] The first step is to take an adult white jade snail with a weight of 35g, and starve it for 1 day. After making it excrete the food in the digestive tract, cut the shell from the shell mouth along the direction of the shell spiral along the suture line until the top of the shell, and then wrap the shell around the shell axis. It should be noted that during the cutting process, it is necessary to constantly remove the broken shells with tweezers, only keep the central shell axis, and then carefully remove the soft body part wound on the shell axis, so as not to damage the inner shell. software structure;
[0020] The second step is to place the cut soft body part in a petri dish, use scissors to cut the body wall wrapped around the viscera mass, lift the body wall, and gently stretch the curled viscera to see the reddish-brown digestion For the digest...
Embodiment 2
[0025] Embodiment 2 The purification method of β-glucuronidase of the present invention comprises the following steps:
[0026] The first step is to take an adult Roman snail with a weight of 45g, starve it for 2 days, make it excrete the food in the digestive tract, cut the shell from the shell mouth along the direction of the shell spiral along the suture line until the top of the shell, and then wrap the shell around the shell axis It should be noted that during the cutting process, it is necessary to constantly remove the broken shells with tweezers, only keep the central shell axis, and then carefully remove the soft body part wound on the shell axis, so as not to damage the inner shell. software structure;
[0027] The second step is to place the cut soft body part in a petri dish, use scissors to cut the body wall wrapped around the viscera mass, lift the body wall, and gently stretch the curled viscera to see the reddish-brown digestion For the digestive glands of the...
Embodiment 3
[0032] Embodiment 3 performance measurement of purified product
[0033] 1. Enzyme Activity Determination Method:
[0034] Use p-nitrophenyl-β-D-glucoside (pNPG) as the substrate for enzymatic hydrolysis, and the p-nitrophenol released after hydrolysis of the substrate has a characteristic absorption peak in the visible light range of 400-420nm, which can be directly in Colorimetric determination between 400-420nm.
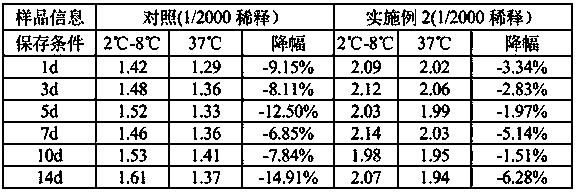
[0035] 2. Enzyme Stability Determination Method: 37°C Thermal Acceleration Determination of Stability.
[0036] 3. Measurement results
[0037] 3.1 Enzyme activity assay results
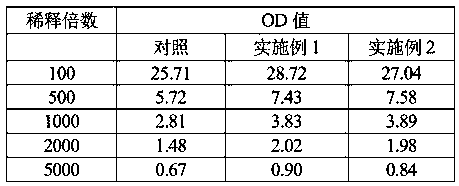
[0038] Measure the β-glucuronidase that embodiment 1 and embodiment 2 purify according to above-mentioned enzyme activity assay method, its result is as shown in table 1 below:
[0039] Table 1
[0040]
[0041] Conclusion: From the data in Table 1, it can be seen that the OD values of the purified β-glucuronidases obtained in Example 1 and Example 2 of the present invention ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com