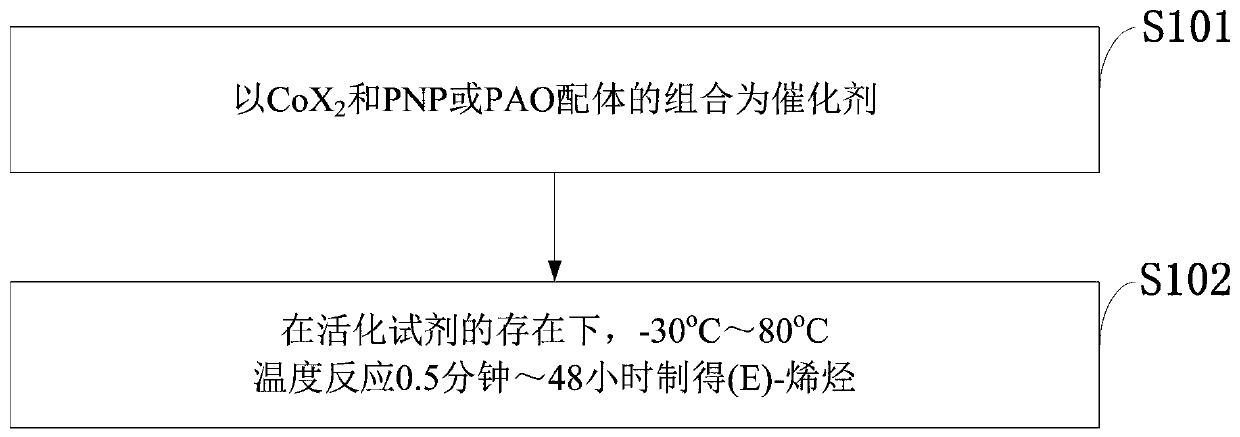
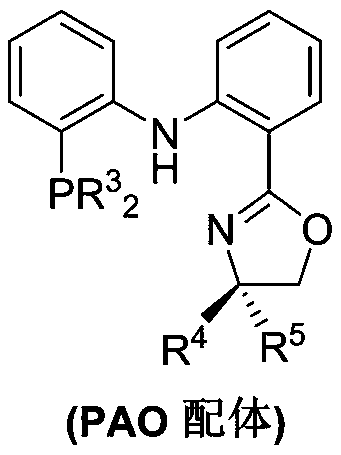
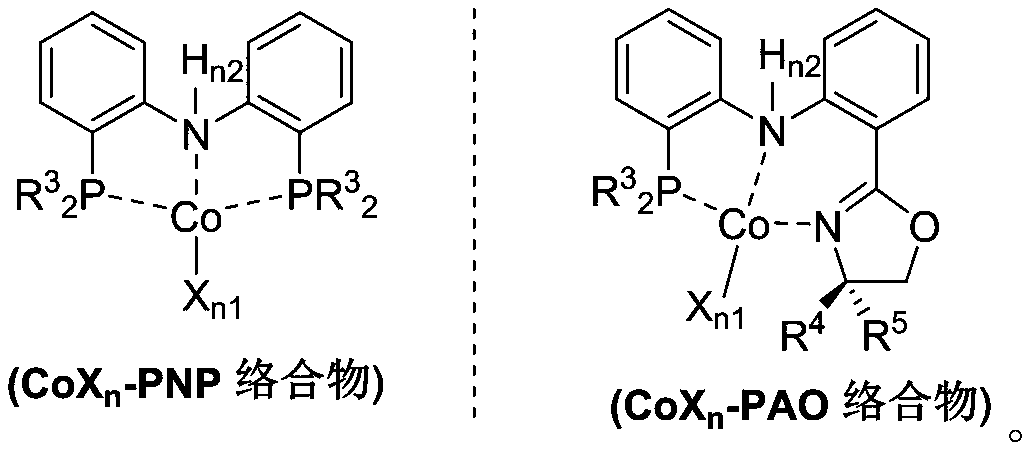
Process for isomerizing and converting (Z)-olefins to (E)-olefins
A technology for isomerization of olefins and olefins, applied in the field of isomerization of -olefins into -olefins, can solve the problems of low catalytic efficiency, small scope of substrate application, large amount of catalyst, etc., and achieve simple reaction process and post-treatment operation , High industrial application prospect and value, high stereoselective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Cobalt-catalyzed isomerization of Z-type olefins into E-type olefins
[0049]
[0050] Operation steps: Add CoCl to a dry reaction test tube at 25°C 2 (0.01mmol), PNP or PAO ligand (0.01mmol), E / Z-mixed alkenes (10mmol), toluene (1mL), injected into sodium triethylborohydride (0.03mmol), then stirred at room temperature for 10 minutes The product was separated by column chromatography.
[0051] Product 1: (E)-Anisene.
[0052]
[0053] Colorless oily liquid, yield 98%, E / Z>99 / 1. 1 H NMR: (400.0MHz, CDCl 3 )δ7.28-7.21(J=6.8,2.2Hz,2H),6.83(dd,J=6.8,2.2Hz,2H),6.34(dq,J=16.0,1.6Hz,1H),6.15-6.03(m ,1H),3.79(s,3H),1.85(dd,J=6.6,1.6Hz,3H).
[0054] Product 2: (E)-Methylisoeugenol.
[0055]
[0056] Colorless oily liquid, yield>99%, E / Z>99 / 1. 1 H NMR: (400.0MHz, CDCl 3 )δ7.30(d, J=8.4Hz, 1H), 6.62(dq, J=16.0, 1.8Hz, 1H), 6.50-6.40(m, 2H), 6.16-6.04(m, 1H), 3.82(s ,3H),3.80(s,3H),1.87(dd,J=6.8,1.8Hz,3H).
[0057] Product 3: (E)-Methyleugenol.
[00...
Embodiment 2
[0120] Example 2: Comparison of regioselective hydrosilation reactions of E / Z mixed olefins and E-olefins
[0121]
[0122] Reaction operation: at 25°C, add [Fe] (0.05mmol), olefin (1mmol), phenylsilane (PhSiH 3) (1.2mmol), tetrahydrofuran (THF) (1mL), injected into ethylmagnesium bromide (EtMgBr) (0.11mmol), then stirred at room temperature for 24 hours and separated by column chromatography to obtain the product.
[0123] Reaction 1 uses E / Z mixed β-methylstyrene as raw material, and the product is a mixture of branched and terminal hydrosilylation products in a ratio of 1.1:1; Reaction 2 uses E-β-methylstyrene as raw material, and the product It is a branched chain hydrosilation product.
[0124] Product 24: phenyl(1-phenylpropyl)silane
[0125]
[0126] Colorless liquid, yield 95%. 1 H NMR (400MHz, CDCl 3 )δ7.44-7.33(m,3H),7.35-7.21(m,4H),7.14(t,J=7.3Hz,1H),7.07(d,J=7.3Hz,2H),4.37-4.24(m ,2H),2.43-2.33(m,1H,),1.99-1.77(m,2H),0.93(t,J=7.2Hz,3H); 13 C NMR (101MHz...
Embodiment 3
[0130] Example 3: Comparison of diastereoselective sulfur-amidation reactions of E / Z mixed olefins and E-olefins
[0131]
[0132] Reaction operation: at 25°C, add olefin (1mmol), p-methylthiophenol (1.5mmol), acetonitrile (CH 3 CN) (2mL), iodine (I 2 ) (0.3mmol) and dimethyl sulfoxide (DMSO) (1.5mmol), then warmed up to 80°C and stirred for 24 hours, and then separated by column chromatography to obtain the product.
[0133] Reaction 1 uses E / Z mixed β-methylstyrene as raw material, and the diastereoselectivity of the sulfur-amidated product is 3:1; Reaction 2 uses E-β-methylstyrene as raw material, sulfur- The diastereoselectivity of the amidation product is greater than 20:1, product 27: N-1-phenyl-2-(p-tolylthio)propyl)acetamide
[0134]
[0135] Yellow solid, yield 78%. 1 H NMR (CDCl 3 ,500MHz):δ7.37-7.31(m,4H),7.32-7.28(m,3H),7.15(d,J=7.1Hz,2H),6.23(d,J=6.2Hz,1H),5.14( dd,J 1 =4.4Hz,J 2 =6.3Hz,1H),3.67-3.57(m,1H),2.35(s,3H),2.02(s,3H),1.20(d,J=6.3Hz,3H); 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com