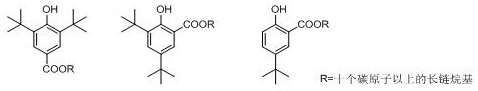
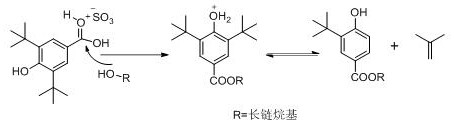
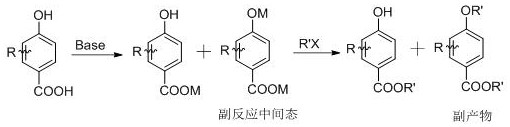
A kind of preparation method of tert-butyl substituted hydroxybenzoate
A technology of hydroxybenzoate and hydroxybenzoate, which is applied in the field of preparation of tert-butyl substituted hydroxybenzoate, and can solve the problem of tert-butyl leaving, esterification of hydroxybenzoate and halogenated hydrocarbon Low yield and other problems, to achieve the effect of reducing by-products, easy purification and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Preparation of n-hexadecyl 5-tert-butyl-2-hydroxybenzoate
[0031] Add 172 grams of 4-tert-butylphenol sodium salt to 342 grams of N,N dimethylformamide, pass through carbon dioxide, react completely at 0.7 MPa, cool down to 15°C, add 13.0 grams of tetrabutylammonium fluoride, slowly drop Add 287 grams of hexadecane chloride, drop it in about 20 minutes, then raise the temperature to 70°C and react for 10 hours until the HPLC detection of 5-tert-butyl-2-hydroxybenzoic acid is less than 1%, and the reaction solvent is recovered by distillation under reduced pressure. The system is viscous, then add 10% hydrochloric acid to adjust the pH to neutral, extract three times with toluene, combine the organic phase and add catalytic amount of white clay to reflux for 2 hours to decolorize, evaporate a large amount of toluene until the system is viscous, add 5 times the volume of methanol and slowly cool down to Crystallize at 10°C, filter the crystals and recrystal...
Embodiment 2
[0032] Embodiment 2: Preparation of n-hexadecyl 5-tert-butyl-2-hydroxybenzoate
[0033] 172 grams of 4-tert-butylphenol sodium salt are introduced into 342 grams of N,N dimethylformamide with carbon dioxide, the reaction is complete at 0.7MPa, the temperature is lowered to 15°C, 13.0 grams of tetrabutylammonium bromide is added, slowly drop Add 287 grams of hexadecane chloride, drop it in about 20 minutes, then raise the temperature to 70°C and react for 10 hours until the HPLC detection of 5-tert-butyl-2-hydroxybenzoic acid is less than 1%, and the reaction solvent is recovered by distillation under reduced pressure. The system is viscous, then add 10% hydrochloric acid to adjust the pH to neutral, extract three times with toluene, combine the organic phase and add catalytic amount of white clay to reflux for 2 hours to decolorize, evaporate a large amount of toluene until the system is viscous, add 5 times the volume of methanol and slowly cool down to Crystallize at 10°C, f...
Embodiment 3
[0034] Embodiment 3: Preparation of n-hexadecyl 5-tert-butyl-2-hydroxybenzoate
[0035]172 grams of 4-tert-butylphenol sodium salt are introduced into 342 grams of N,N dimethylformamide with carbon dioxide, and the reaction is complete under a pressure of 0.7MPa. The temperature is lowered to 15°C, and 26.0 grams of tetrabutylammonium bromide is added, slowly Add 310 grams of hexadecane chloride dropwise, and the dropwise addition is completed in about 20 minutes, then raise the temperature to 75°C and react for 10 hours until the HPLC detection of 5-tert-butyl-2-hydroxybenzoic acid is less than 1%, and the reaction solvent is recovered by distillation under reduced pressure When the system is viscous, add 10% hydrochloric acid to adjust the pH to neutral, extract three times with toluene, combine the organic phase and add a catalytic amount of white clay to reflux for 2 hours to decolorize, evaporate a large amount of toluene until the system is viscous, then add 5 times the v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com