The preparation method of 16α-hydroxyprednisolone
A technology of hydroxyprednisolone and dimethyl hydantoin, applied in the field of medicinal chemistry, can solve the problems of multiple impurities, difficult purification, low yield and the like, and achieve high reactivity, increase reaction yield, and reduce reaction temperature. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
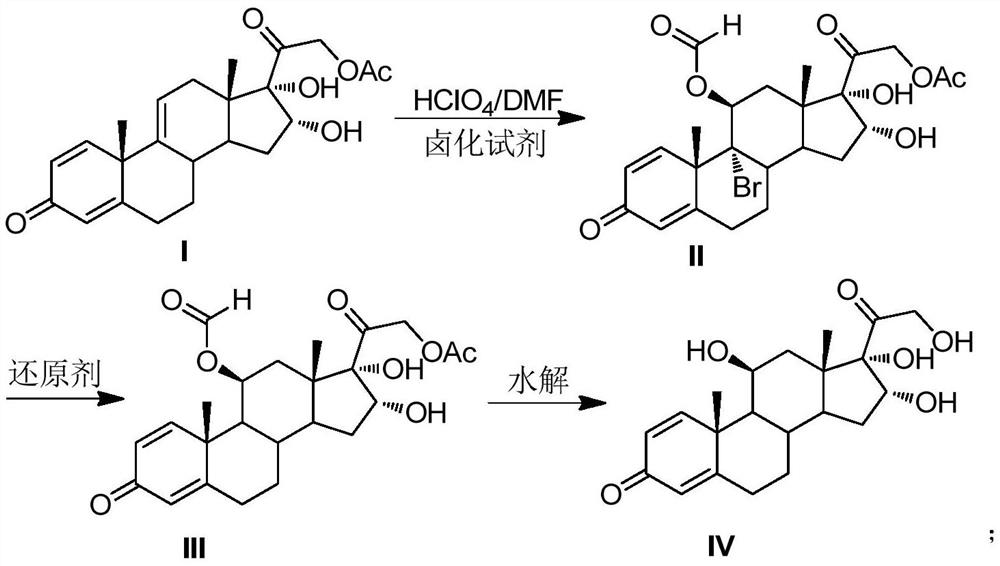
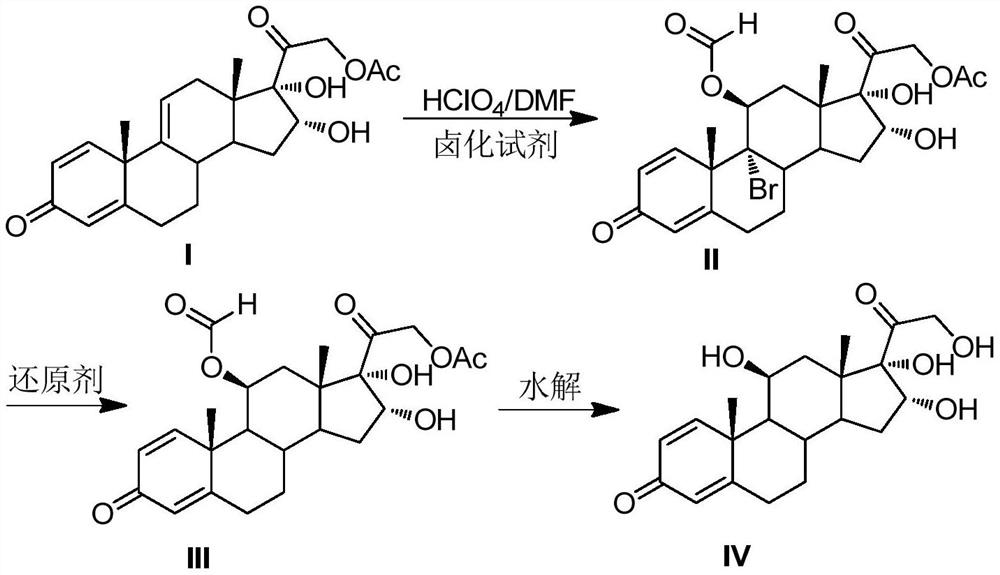
[0021] Preparation of 11,16,17,21-tetrahydroxypregna-1,4-diene-9-bromo-3,20-dione-11-formate-21-acetate compound Ⅱ
[0022] Put DMF200mL, 16,17,21-trihydroxypregna-1,4,9-triene-3,20-dione-21-acetate compound I40.0g (0.10mol, 1.0 equiv), under the protection of nitrogen, cool down to 0°C, add 14.3g (0.05mol, 0.5equiv) of 1,3-dibromo-5,5-dimethylhydantoin, stir to dissolve, slowly add perchloric acid 4.0 mL (0.05mol, 0.5equiv), pay attention to control the reaction temperature at -5℃~5℃. After the addition was completed, TLC detected that the reaction was complete. Add 50 mL of 4% sodium sulfite solution dropwise to terminate the reaction. The reaction solution was poured into 1000 mL of ice water, and the white solid II was obtained by water analysis and suction filtration, and dried to obtain 51.5 g of the product, with a molar yield of 98.2%. , HPLC purity 99.1%.
[0023] Preparation of 11,16,17,21-tetrahydroxypregna-1,4-diene-3,20-dione-11-formate-21-acetate compound III ...
Embodiment 2
[0028] Preparation of compound Ⅱ
[0029] Put 200mL of DMF and 40.0g (0.10mol, 1.0equiv) of compound I solid into a dry and clean reaction bottle. ) after stirring to dissolve, slowly add perchloric acid 7.9mL (0.10mol, 1.0equiv) dropwise, pay attention to control the reaction temperature at -5°C ~ 5°C. After the addition was complete, TLC detected that the reaction was complete. Add 50mL of 4% sodium sulfite solution dropwise to terminate the reaction, pour the reaction solution into 1000mL of ice water, water analysis and suction filtration to obtain a white solid compound II, and dry at 50°C to obtain 51.4g of the product, mol Yield 98.0%, HPLC purity 99.2%.
[0030] Preparation of compound III
[0031] Add compound II51.4g (0.098mol, 1.0equiv), CrCl 2 24.6g (0.20mol, 2.0equiv), N,N-dimethylformamide 500mL, finally add thioglycolic acid 18.4g (0.20mol, 2.0equiv), control the reaction temperature 5 ~ 15 ℃, TLC detection of complete reaction, the reaction The solution wa...
Embodiment 3
[0035] Preparation of compound Ⅱ
[0036] Put 200mL of DMF and 40.0g (0.10mol, 1.0equiv) of compound I solid into a dry and clean reaction bottle. ) after stirring to dissolve, slowly add perchloric acid 9.5mL (0.12mol, 1.2equiv) dropwise, pay attention to control the reaction temperature 0 ~ 5 ℃. After the addition was complete, TLC detected that the reaction was complete. Add 50mL of 4% sodium sulfite solution dropwise to terminate the reaction. The reaction solution was poured into 1000mL of ice water, water analysis and suction filtration to obtain a white solid compound II, which was dried at 50°C to obtain 46.2g of the product, molar yield The yield was 96.3%, and the HPLC purity was 99.1%.
[0037] Preparation of compound III
[0038] Add 46.2g (0.0963mol, 1.0equiv) of compound II, 31.5g (0.48mol, 5.0equiv) of zinc powder, 2.56g (0.01mol, 0.1equiv) of chromium chloride hexahydrate, 300 mL of N-methylpyrrolidone, and finally 2.04 g (0.19 mol, 2.0 equiv) of mercaptopro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com