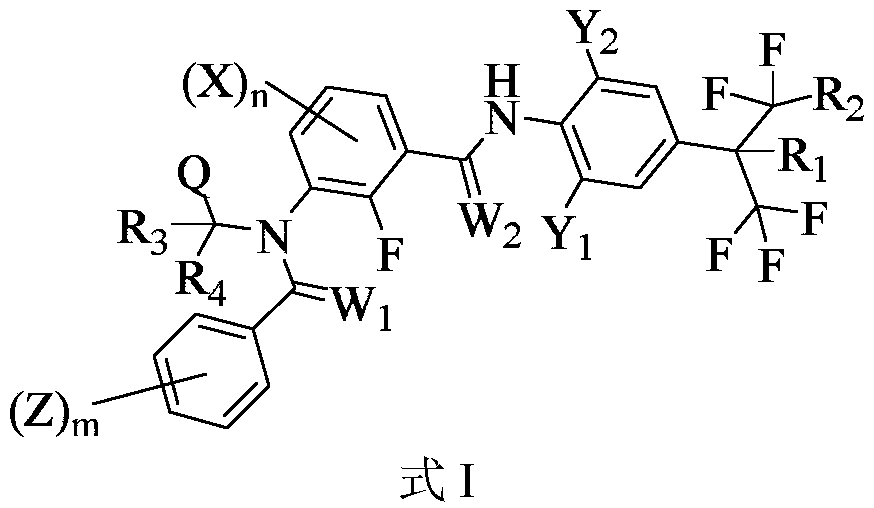
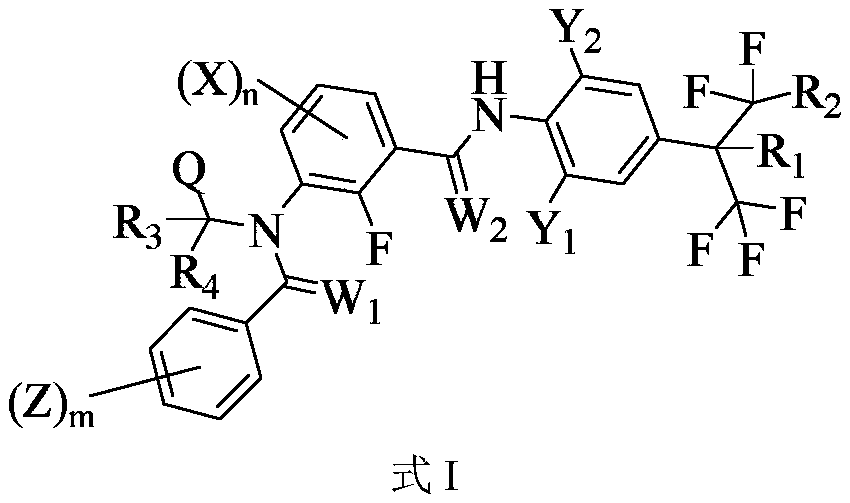
Medicine composition containing m-diamide compound and application of medicine composition
A technology for metadiamide and compound, which is applied in the field of pharmaceutical compositions containing metadiamide compounds, can solve the problem of incomplete insecticidal activity and the like, achieve good effect, reduce damage to plants and humans, and reduce drug residues. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] N-[2-Bromo-4-(1,1,1,2,3,3,3-heptafluoroprop-2-yl)-6-(trifluoromethyl)phenyl]-3-[N- (Cyclopropylmethyl)-benzamido]-2-fluorobenzamide (compound number 1):
[0085] (1) Synthesis of 2-fluoro-[3-(cyclopropylmethyl)amino]benzoic acid methyl ester
[0086]
[0087] Add methyl 2-fluoro-3-aminobenzoate (20g, 118.23mmol), bromomethylcyclopropane (20.75g, 153.70mmol), potassium carbonate (21.24g, 153.70mmol), N, N-dimethylformamide (200 mL) was stirred under reflux for 16 h. When TLC monitored that the reaction did not proceed, the heating was turned off to terminate the reaction. After the reaction solution was cooled to room temperature, water (200 mL) was added to the reaction solution, extracted with ethyl acetate (100 mL), the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was washed with Purified by column chromatography (eluent is petroleum ether: ethyl acetate = 10:1), the produ...
Embodiment 2
[0100] N-[2-Bromo-4-(1,1,1,2,3,3,3-heptafluoroprop-2-yl)-6-trifluoromethoxyphenyl]-3-[N-( Synthesis of cyclopropylmethyl)benzamido]-2-fluorobenzamide (compound number 3)
[0101]
[0102] Add 2-fluoro-3-(N-(cyclopropylmethyl)benzamide)benzoic acid (0.50g, 1.60mmol), toluene (6mL), thionyl chloride (1.07g, 9.00mmol) to the reaction flask successively ), the reaction was stirred under reflux for 2 h, the toluene was concentrated under reduced pressure, and the concentrated residue was dissolved in tetrahydrofuran (3 mL) for use.
[0103] 2-Bromo-4-heptafluoroisopropyl-6-trifluoromethoxyaniline (0.68g, 1.60mmol) was dissolved in tetrahydrofuran (4mL), and 2M lithium diisopropylamide was added dropwise at -70°C Tetrahydrofuran solution (0.96mL, 1.93mmol) was added dropwise after 5min with the tetrahydrofuran solution to be used in the next step, stirred at -70°C for 30min, then raised to room temperature and continued to stir for 30min. Thin-layer chromatography (TLC) monitor...
Embodiment 3
[0106] N-[2-Bromo-4-(1,1,1,2,3,3,3-heptafluoroprop-2-yl)-6-(trifluoromethyl)phenyl]-3-[N- Preparation of (cyclopropylmethyl)-4-cyanobenzamido]-2-fluorobenzamide (compound number 4):
[0107] (1) Synthesis of methyl 2-fluoro-3-[N-(cyclopropylmethyl)-4-cyanobenzamido]benzoate
[0108]
[0109] Add 4-cyanobenzoic acid (0.80g, 5.38mmol), toluene (6mL), and thionyl chloride (3.2g, 26.9mmol) to the reaction flask successively, stir the reaction under reflux for 2h, and concentrate the toluene under reduced pressure , the concentrated residue was dissolved in THF (3 mL) for use. Dissolve 2-fluoro-[3-(cyclopropylmethyl)amino]methyl benzoate (1.0g, 4.48mmol) in tetrahydrofuran (6mL), add triethylamine (0.74g, 5.38mmol), dropwise add 4 -The tetrahydrofuran solution of cyanobenzoyl chloride was stirred at room temperature for 4h. When TLC monitors that the reaction no longer proceeds, the reaction is terminated. Water (20 mL) was added to the reaction solution, extracted with ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com