Thiphenanthridinone derivative, synthetic method thereof and electronic device containing thiaphenanthridinone derivative
A technology of thiaphenanthridone and heterophenanthridone is applied in the fields of electronic devices, thiaphenanthridone derivatives and synthesis thereof, and achieves the effects of easy availability of raw materials, enhanced durability and wide industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
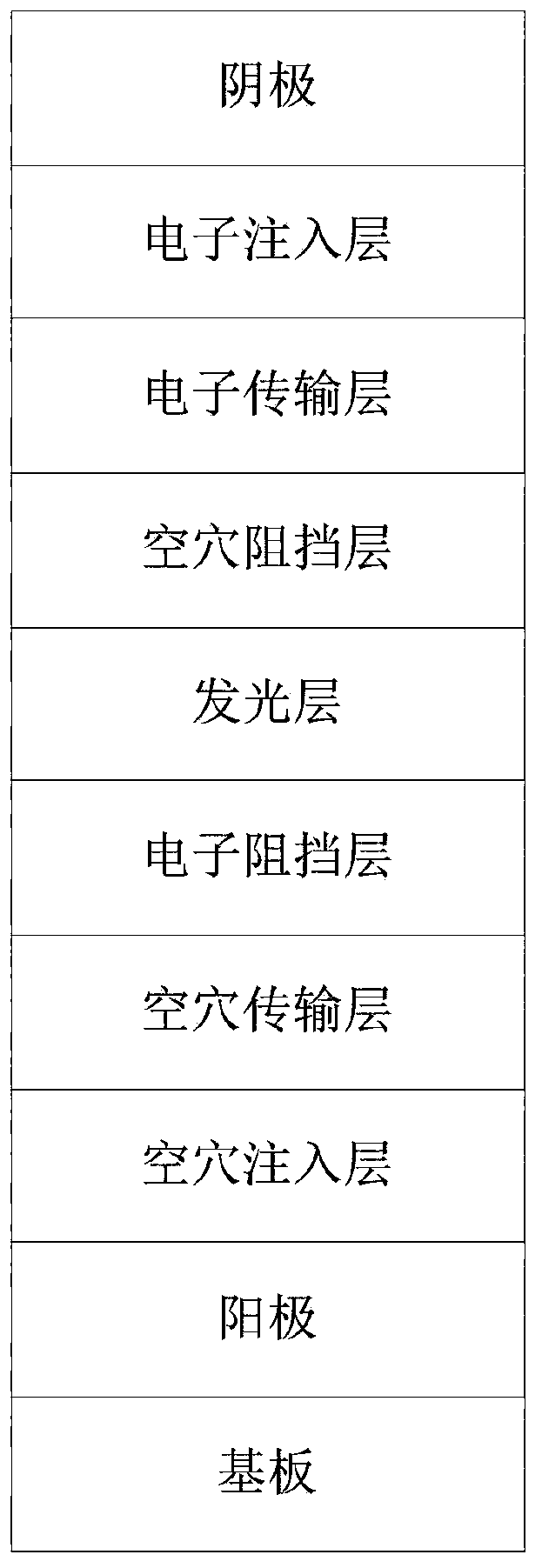
Image
Examples
Embodiment 1
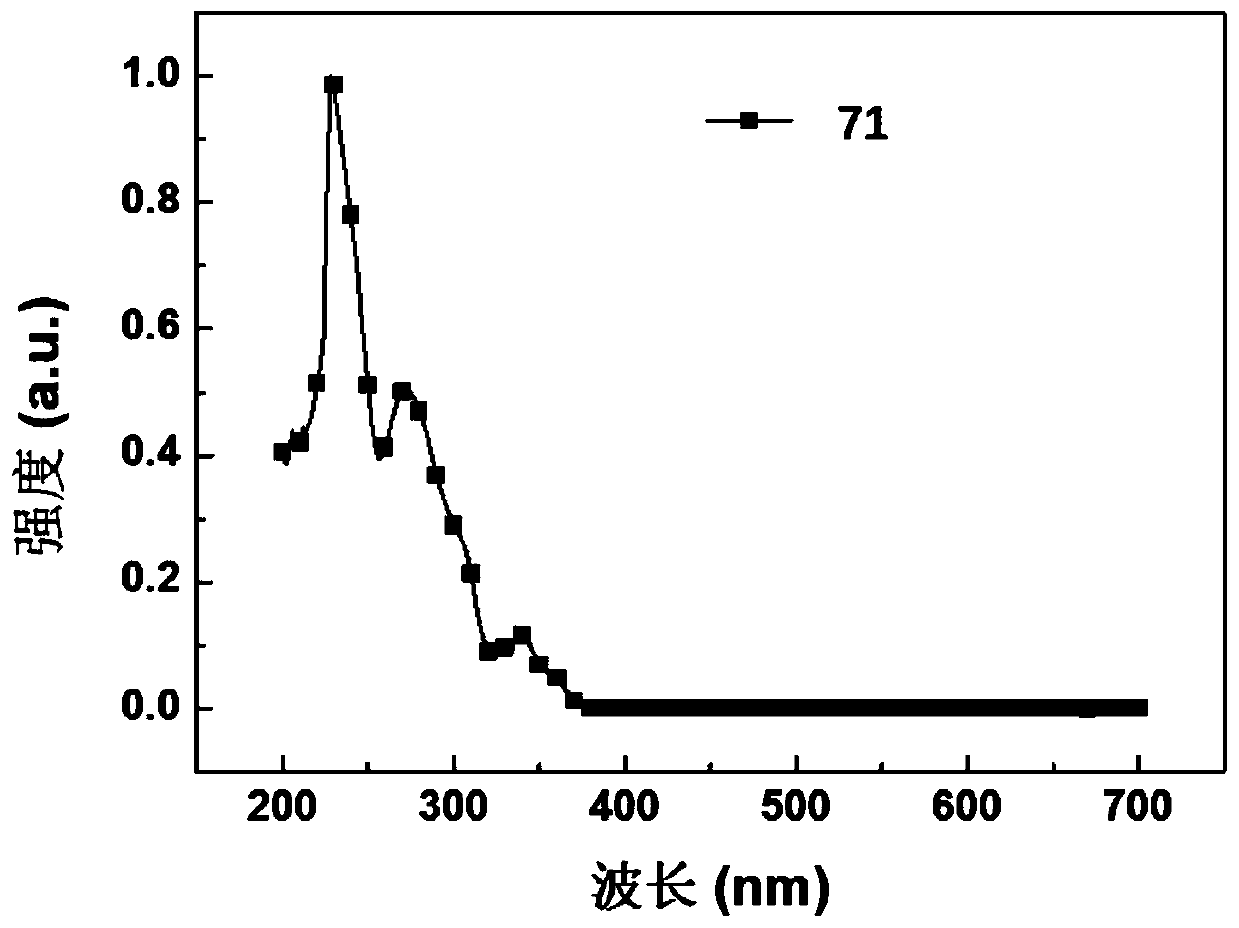
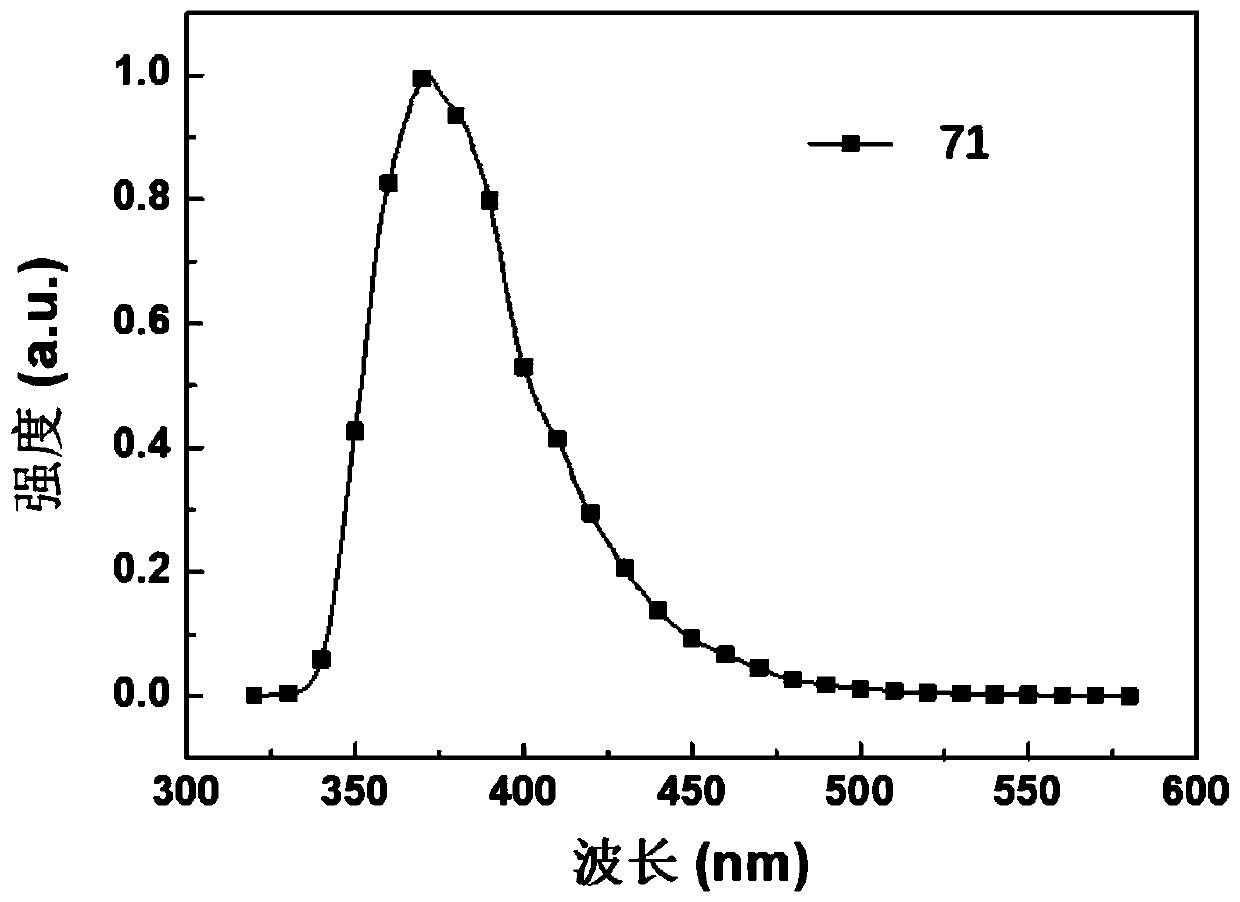
[0131] Embodiment 1: the synthesis of compound 71
[0132] (Synthesis of Intermediate 1)
[0133] The synthetic route of intermediate 1 is as follows:
[0134]
[0135] Under nitrogen atmosphere, add 100mL dichloromethane, methylamine (25mmol, 775mg) and triethylamine (21mmol, 2.94mL) successively to a dry and clean 250mL single-necked bottle, and cool the system to 0°C, dropwise Add [1,1'-biphenyl]-2-sulfonyl chloride (20mmol, 5.06g), continue to stir under ice bath for 15min after the dropwise addition, remove the ice bath, and continue to stir overnight at room temperature, to be reacted After completion, a small amount of water was added to quench the reaction, the organic phase was washed three times with water, and the solvent was evaporated to obtain a crude product, which was further purified by column chromatography (350 mesh silica gel, and the eluent was petroleum ether:dichloromethane=2:1 , V / V) to obtain 4.5 g of white crystalline solid with a yiel...
Embodiment 2
[0144] Embodiment 2: the synthesis of compound 72
[0145] (Synthesis of compound 72)
[0146] The synthetic route of compound 72 is as follows:
[0147]
[0148] Under nitrogen, add intermediate 2 (10mmol, 3.24g), anhydrous potassium carbonate (20mmol, 2.76g), 4-(bis(4-tert-butylphenyl)amino) Phenylboronic acid (12mmol, 4.81g), tetrakis(triphenylphosphine palladium) (0.2mmol, 230mg) and 100mL mixed solvent (toluene:water:ethanol=5:1:1, V / V), the system was heated to reflux and React overnight under reflux. After the reaction is completed, stop heating, and the reaction system is cooled to room temperature by itself. Pour the reaction liquid into about 200 mL of water, extract with dichloromethane, dry the organic phase with anhydrous sodium sulfate, and concentrate under reduced pressure. , and further purified by column chromatography (350 mesh silica gel, eluent: petroleum ether:dichloromethane=3:1, V / V) to obtain 5.1 g of white solid with a yield of 85%. M...
Embodiment 3
[0149] Embodiment 3: the synthesis of compound 172
[0150] (Synthesis of Intermediate 3)
[0151] The synthetic route of intermediate 3 is as follows:
[0152]
[0153] Add intermediate 1 (20mmol, 4.94g), dibromohydantoin (60mmol, 17.2g) and 150mL water successively to a clean 250mL single-necked bottle, and the system is gradually warmed up to 100°C, and reacted overnight at this temperature, and then After the reaction, the heating was stopped, extracted three times with dichloromethane, and the organic phase was dried and concentrated to obtain the corresponding crude product, which was further purified by column chromatography (350 mesh silica gel, eluent was petroleum ether:ethyl acetate=8:1 , V / V) to obtain 6.4 g of white solid, yield 80%. MS(EI): m / z: 403.02[M + ]. Anal.calcd for C 13 h 9 Br 2 NO 2 S (%): C 38.74, H 2.25, N 3.47; found: C 38.70, H 2.28, N 2.43.
[0154] (Synthesis of compound 172)
[0155] The synthetic route of compoun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com