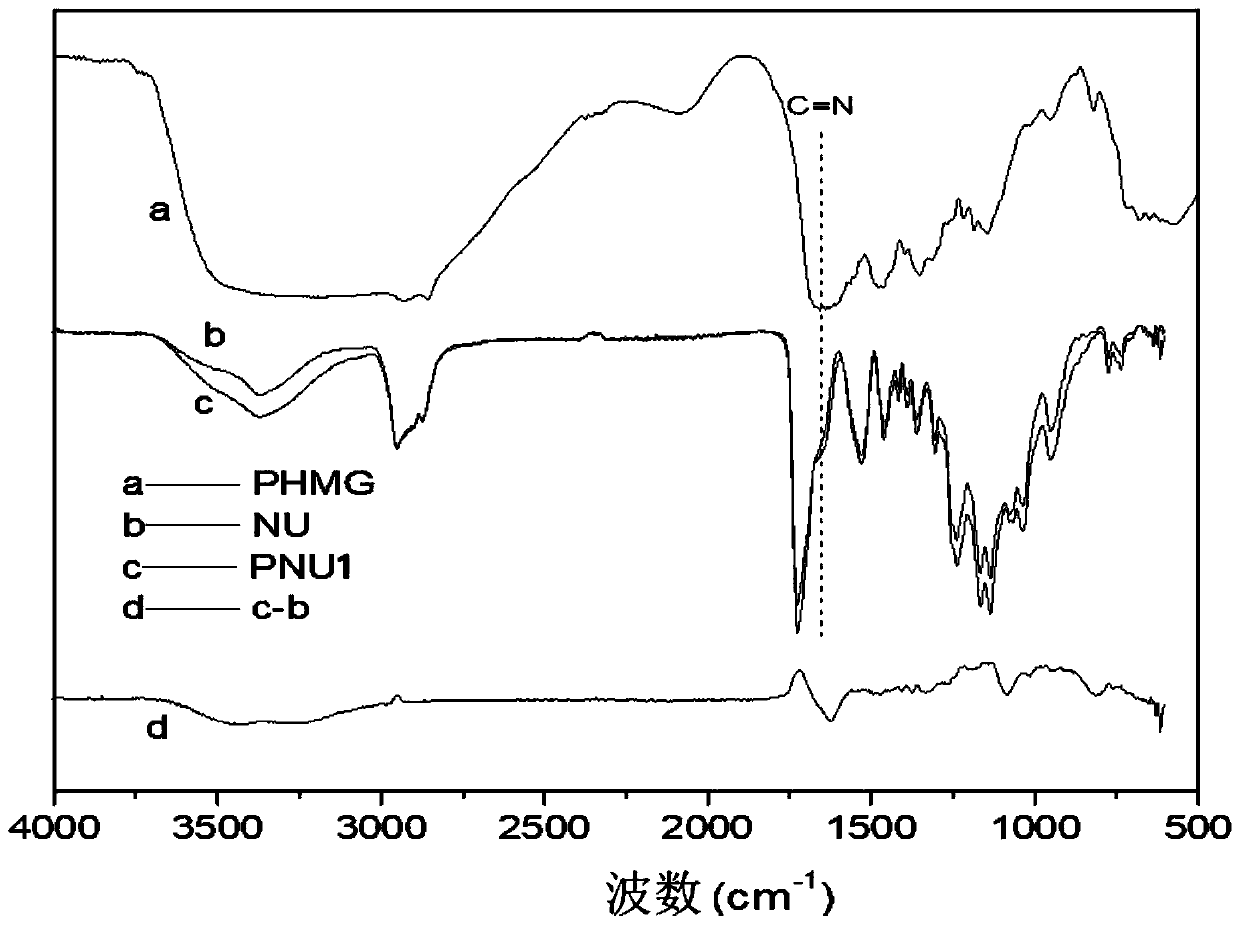
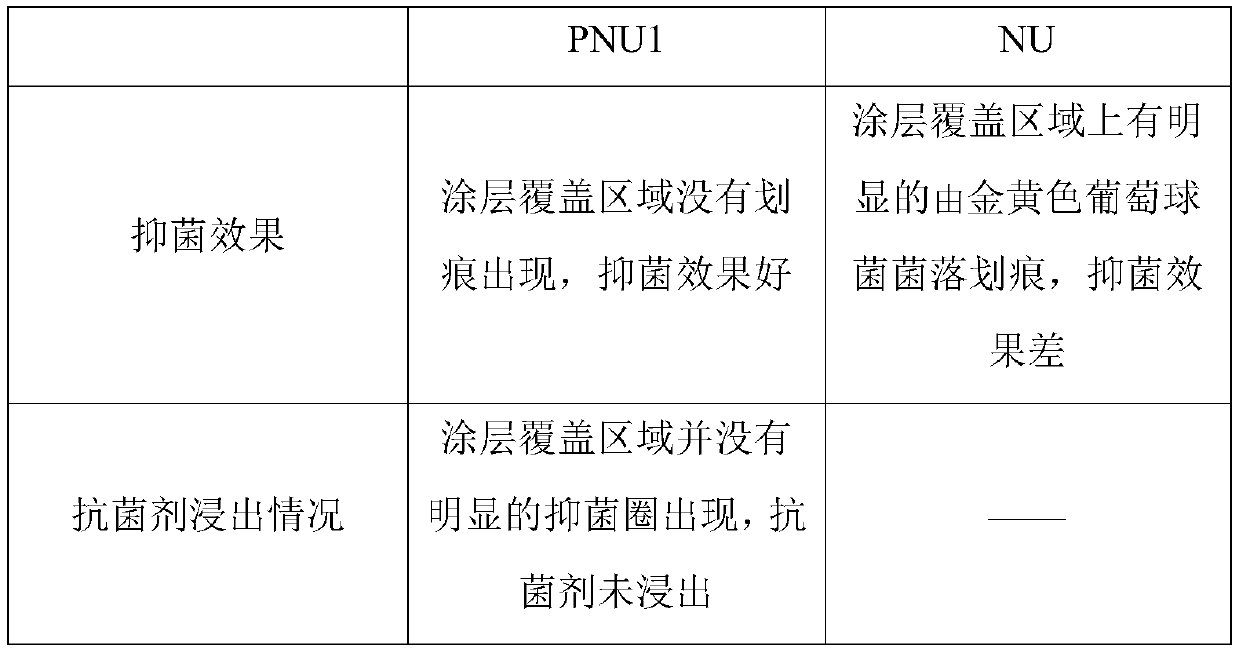
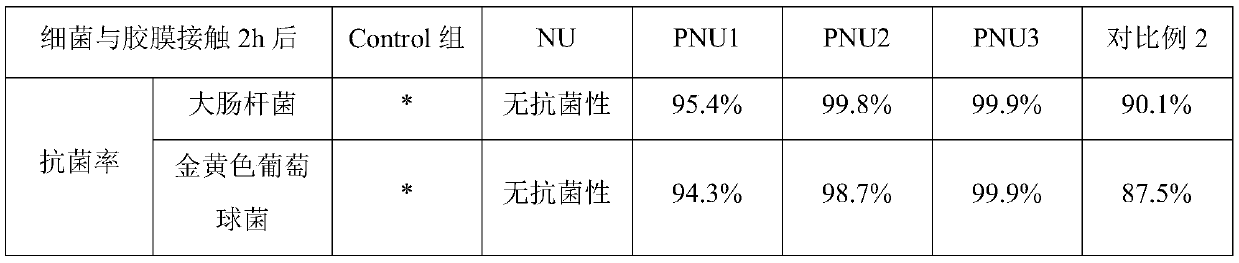
Antibacterial non-ionic waterborne polyurethane and preparation method thereof
A water-based polyurethane and non-ionic technology, which is applied in antifouling/underwater coatings, coatings, paints containing biocide, etc., can solve the problems of low antibacterial durability and affect the stability of water-based polyurethane emulsion, and achieve antibacterial performance improvement , Improve the production and living environment, and prevent the spread of bacteria and viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of UV-curable non-ionic water-based polyurethane emulsion: R=1.5, 40g of polycaprolactone diol (PCL1000) and 7g of Ymer-N120 were added to the reactor for vacuum dehydration at 120°C for 2h, after cooling to 50°C, 15.6g was added Isophorone diisocyanate (IPDI) and 0.0027g stannous octoate were reacted at 80°C for 4h, adding an appropriate amount of acetone during the reaction to adjust the viscosity to 16.0mPa·s-30.0mPa·s; cooling down to 50°C, adding 6g of methacrylic acid Hydroxyethyl ester (HEMA), react at 55°C for 5h; cool down to 50°C, add 160g of deionized water for high-speed shear emulsification for 20min, and obtain UV-curable non-ionic water-based polyurethane emulsion after removing acetone;
[0043] Preparation of antibacterial non-ionic water-based polyurethane: Weigh 10 g of UV-curable non-ionic water-based polyurethane emulsion, add 0.09 g of photoinitiator 2-hydroxy-2-methyl-1-phenylacetone and 0.03 g of double-bond functionalized PHMG, Sonic...
Embodiment 2
[0046] Preparation of UV-curable non-ionic water-based polyurethane emulsion: R=1.5, add 40g of polycaprolactone diol (PCL1000) and 7g of Ymer-N120 into the reactor for vacuum dehydration at 120°C for 2h; after cooling down to below 50°C, add 15.6 g of isophorone diisocyanate (IPDI) and 0.0027g of stannous octoate, reacted at 80°C for 4 hours, during the reaction, add an appropriate amount of acetone to adjust the viscosity to 16.0mPa·s~30.0mPa·s; after cooling down to below 50°C, add 6g Hydroxyethyl methacrylate (HEMA), react at 55°C for 5h; cool down to below 50°C, add 160g of deionized water for high-speed shear emulsification for 20min, remove acetone to obtain UV-curable non-ionic water-based polyurethane emulsion;
[0047] Preparation of antibacterial non-ionic water-based polyurethane: Weigh 10 g of UV-curable non-ionic water-based polyurethane emulsion, add 0.09 g of photoinitiator 2-hydroxy-2-methyl-1-phenylacetone and 0.06 g of double-bond functionalized PHMG, Sonica...
Embodiment 3
[0053] Preparation of UV-curable non-ionic water-based polyurethane emulsion: R=1.5, 40 g of polycaprolactone diol (PCL1000) and 7 g of Ymer-N120 were added to the reactor for vacuum dehydration at 120 ° C for 2 h; the temperature was lowered to 50 ° C, and 15.6 g of iso Phorone diisocyanate (IPDI) and 0.0027g stannous octoate were reacted at 80°C for 4 hours, during the reaction, an appropriate amount of acetone was added to adjust the viscosity to 16.0mPa·s~30.0mPa·s; the temperature was lowered to 50°C, and 6g of methacrylic acid hydroxyl Ethyl ester (HEMA), react at 55°C for 5h; cool down to 50°C, add 160g of deionized water for high-speed shear emulsification for 20min, and obtain UV-curable non-ionic water-based polyurethane emulsion after removing acetone;
[0054] Preparation of antibacterial non-ionic water-based polyurethane: Weigh 10 g of UV-curable non-ionic water-based polyurethane emulsion, add 0.09 g of photoinitiator 2-hydroxy-2-methyl-1-phenylacetone and 0.09 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com