Method for production of cesium salt
A kind of cesium salt, said technology, applied in the field of purification of cesium compounds, can solve problems such as the corrosion effect of drilling equipment materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] This embodiment illustrates application of a method of the present invention to produce cesium formate through one-step reaction and to purify cesium formate.
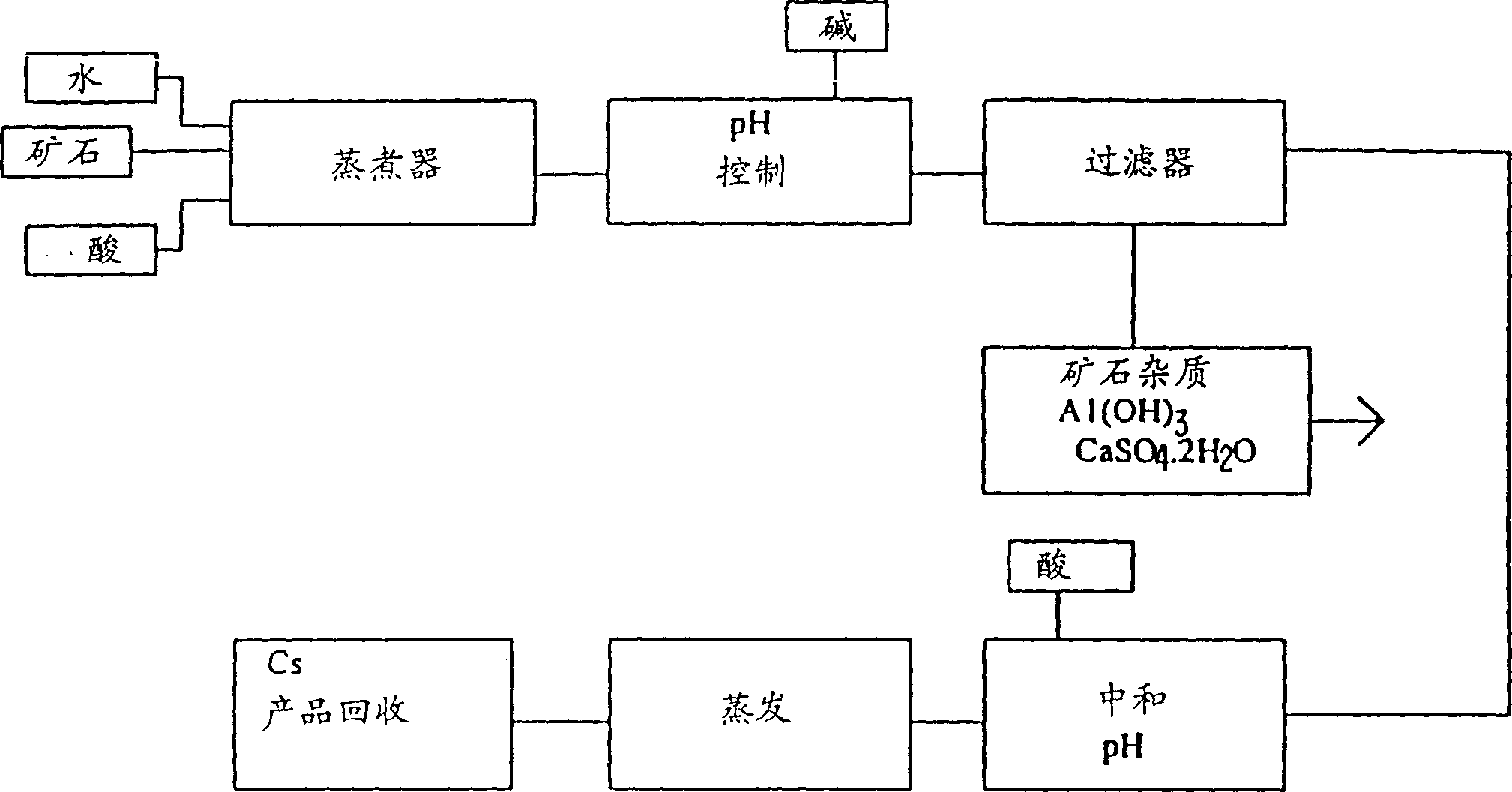
[0128] Take a 4-liter glass beaker and fill it with 444 grams of cesium garnet ore powder ground to a nominal -200 mesh, 670 milliliters of water, and 310 milliliters of 98% (by weight) sulfuric acid. This represents an excess of approximately 82% acid over the stoichiometric amount required to dissolve alkali metals and aluminum from the ore. The mixing was continued while heating the mixture at about 115°C for 16 hours. Add water to maintain extraction volume.
[0129] After 16 hours, the slurry was diluted with water to a volume of 2200 ml, heated to about 80-90°C, and then cooled to room temperature. Decant 940 ml of clear liquid to remove most of the remaining unreacted sulfuric acid. Then add 900 milliliters of water to re-beat the waste ore and crystallized cesium alum, and then heat the re-beating sol...
Embodiment 2
[0146] Present embodiment also illustrates the process of applying the method of the present invention to produce cesium formate and purifying cesium formate.
[0147] Take a 4-liter glass beaker and put 444 grams of ground-200 mesh powdered cesium garnet, 670 milliliters of water, and 310 milliliters of 98% (weight) sulfuric acid. The mixture was mixed and heated to about 115°C for 16 hours. Add water to maintain extraction volume.
[0148] After 16 hours, the slurry was diluted with water to a volume of 2200-2500 ml, reheated, and then cooled to room temperature. The supernatant 1135 ml was decanted to remove most of the remaining sulfuric acid. Then add 800 milliliters of water to re-beat the remaining cesium alum and waste ore, and heat to about 70°C under stirring.
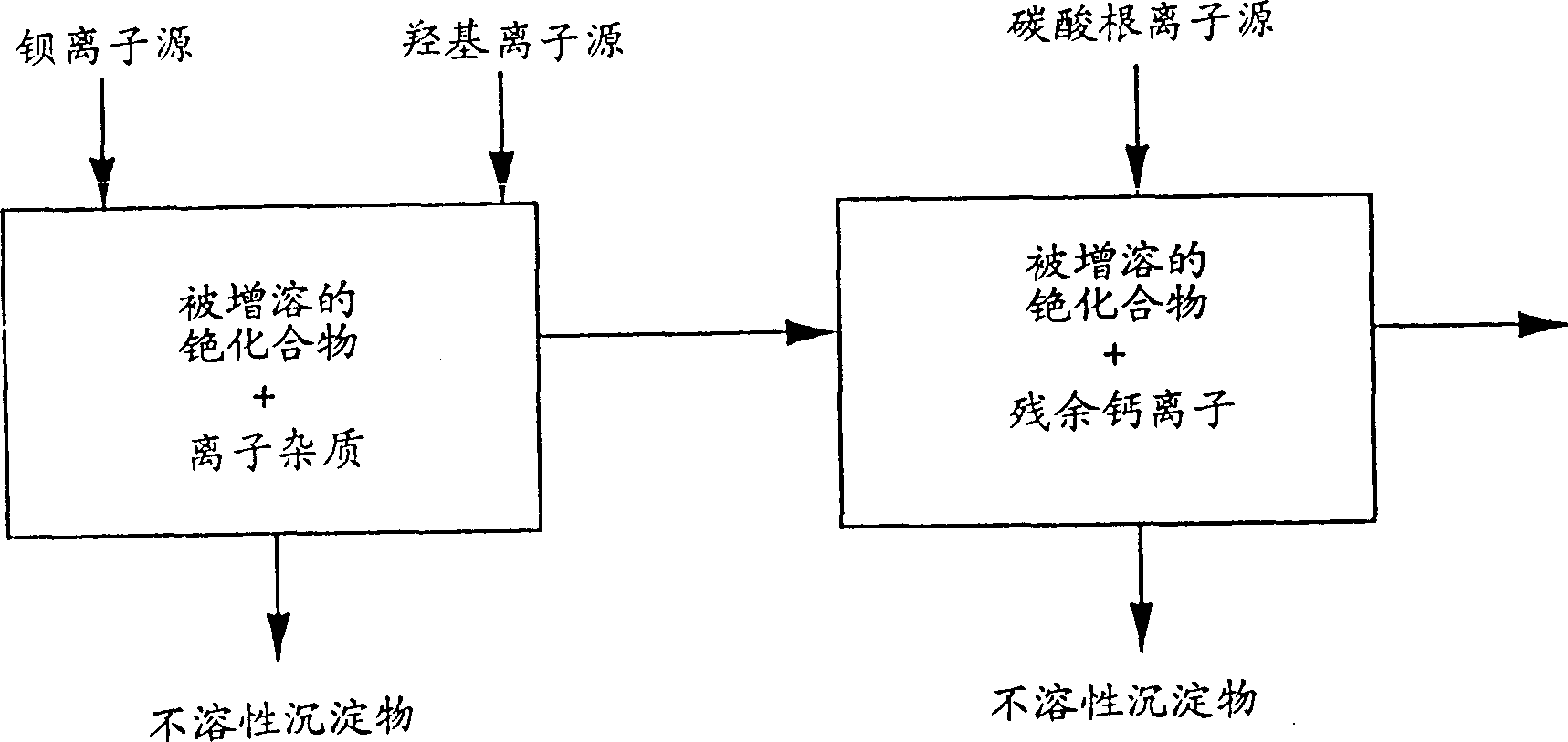
[0149] Then add the slaked lime slurry prepared by 150 grams of calcium oxide in 500 milliliters of water, and the resulting pH value is 7-8. The slurry was mixed at 90°C for 1.5 hours and cooled to 60°C....
Embodiment 3
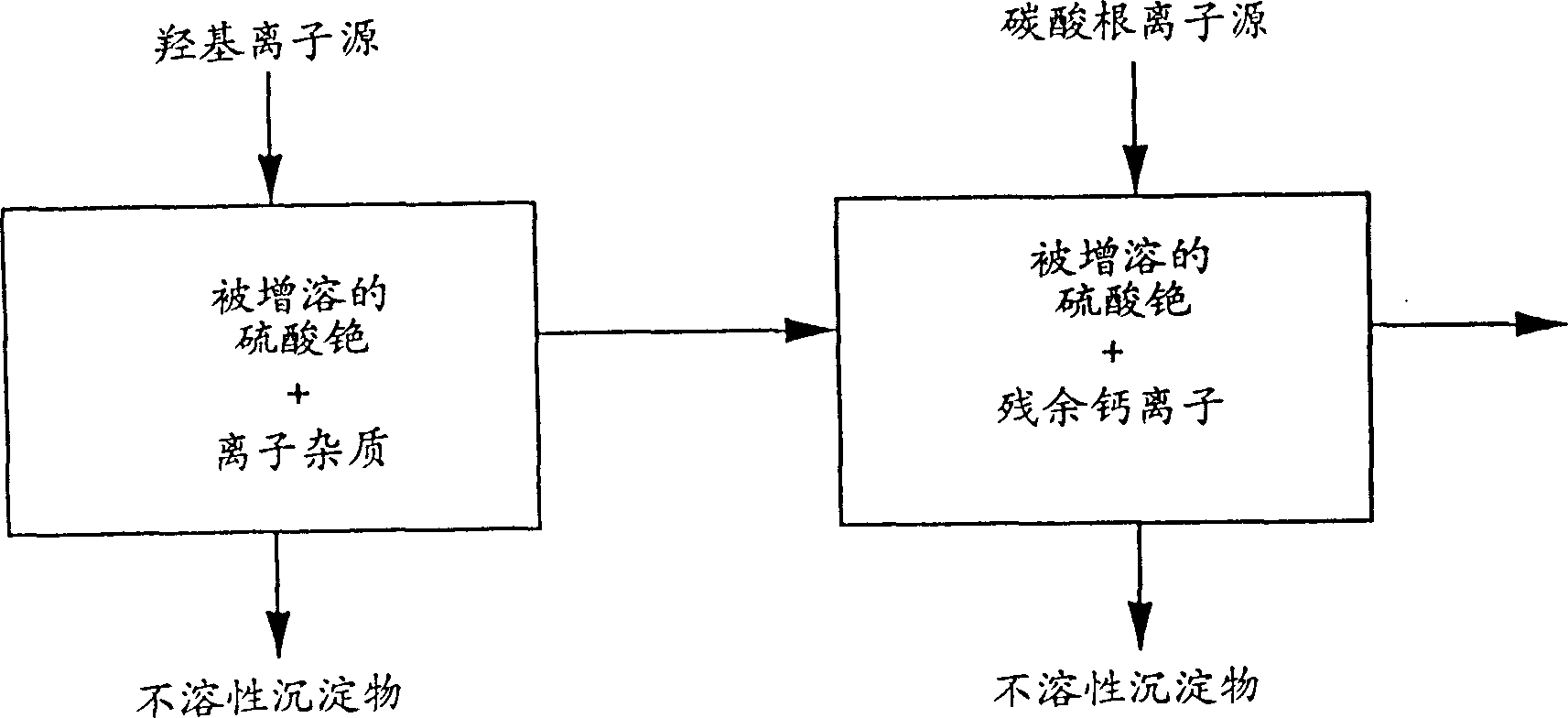
[0167] The present embodiment illustrates the process of utilizing the inventive method to produce cesium sulfate and purify cesium sulfate.
[0168] Get 4 liters of glass beakers, put into 444 grams and grind into a 200 mesh powdery cesium garnet, 670 milliliters of water, 310 milliliters of 98% (weight) sulfuric acid. The mixture was mixed and heated to about 115°C for 16 hours. Add water to maintain the extraction volume so that the solid-to-liquid ratio is maintained at an acceptable level. After 16 hours, the slurry was diluted with water to a volume of 1800 ml, reheated, and then recooled to room temperature. Decant 960 ml of the clear liquid to remove most of the remaining sulfuric acid. Then add about 1000 milliliters of water to re-beat the remaining cesium alum and waste ore, then heat the re-beating slurry under stirring, and then heat to about 80°C. Take slaked lime slurry consisting of 160 grams of calcium oxide and about 300 milliliters of water, and add it to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com