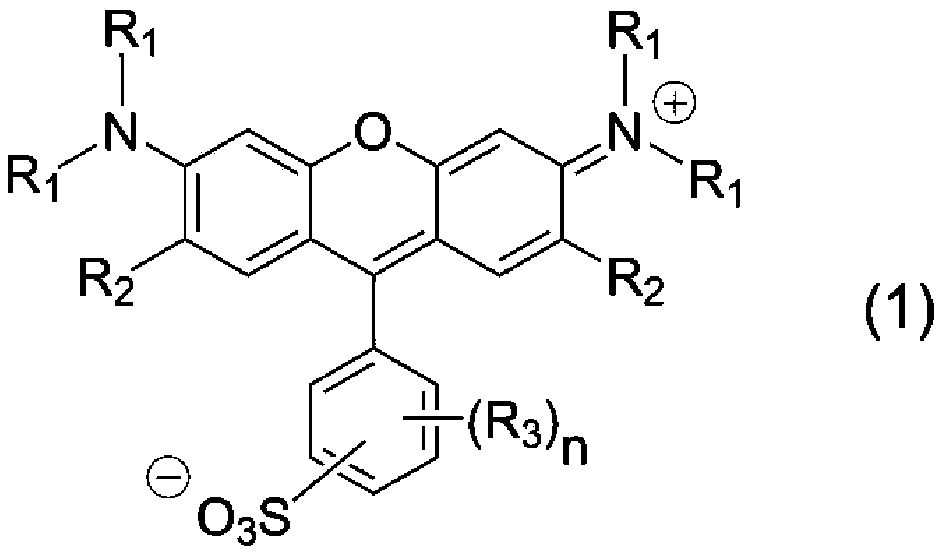
Xanthene compound, pigment composition, color photoresist, color filter, and ink composition for inkjet
A technology of dibenzopyran and pigment composition, applied in the field of dibenzopyran compounds, can solve problems such as adverse effects, insufficient pigment dispersion, unstable quality of pigment dispersion liquid, etc., and achieve the effect of easy manufacture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1 (synthesis of the compound represented by No.1 of the specific example)
[0100] After dissolving 30.0 parts of C.I.Acid Red 52 in 300 parts of water in a reaction vessel, add 5.7 parts of calcium chloride to the solution at 20 to 30°C, and stir at the same temperature for one hour. After filtering the precipitated crystals, the obtained wet cake was dried in a hot air dryer at 80° C. to obtain 23.6 parts of the compound represented by No. 1 above. The maximum absorption wavelength λmax of this compound is 565 nm (aqueous solution).
Embodiment 2
[0101] Example 2 (synthesis of the compound represented by No. 2 of the specific example)
[0102] An aqueous solution obtained by dissolving 30.0 parts of C.I.Acid Red 52 in 300 parts of water in a reaction vessel, and then dissolving 23 parts of 18-amino-8,11,13-arbietatriene in 100 parts of 3.3% acetic acid aqueous solution Spend 30 minutes at 20 to 25° C. into the solution, and stir at the same temperature for one hour. After filtering the precipitated crystals, the obtained wet cake was dried in a hot air drier at 80° C. to obtain 32.0 parts of the compound represented by No. 2 above. The maximum absorption wavelength λmax of this compound is 565 nm (aqueous solution).
Synthetic example 1
[0103] Synthesis Example 1 (Preparation of Binder Resin A)
[0104] Put 160 parts of methyl ethyl ketone, 10 parts of methacrylic acid, 33 parts of benzyl methacrylate, and 1 part of α,α'-azobis(isobutyronitrile) into a 500 mL four-necked flask, and make nitrogen gas while stirring. Flow into the flask for 30 minutes. Then the temperature was raised to 80° C. and maintained at 80 to 85° C. with stirring for 4 hours. After completion of the reaction, it was cooled to room temperature to obtain a colorless, transparent and uniform binder resin solution. The binder resin solution was precipitated in a mixed solution of isopropanol and water at a ratio of 1:1, filtered, and the solid was taken out and dried to obtain binder resin A. The obtained binder resin A had a polystyrene-equivalent weight average molecular weight of 18,000 and an acid value of 152.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com