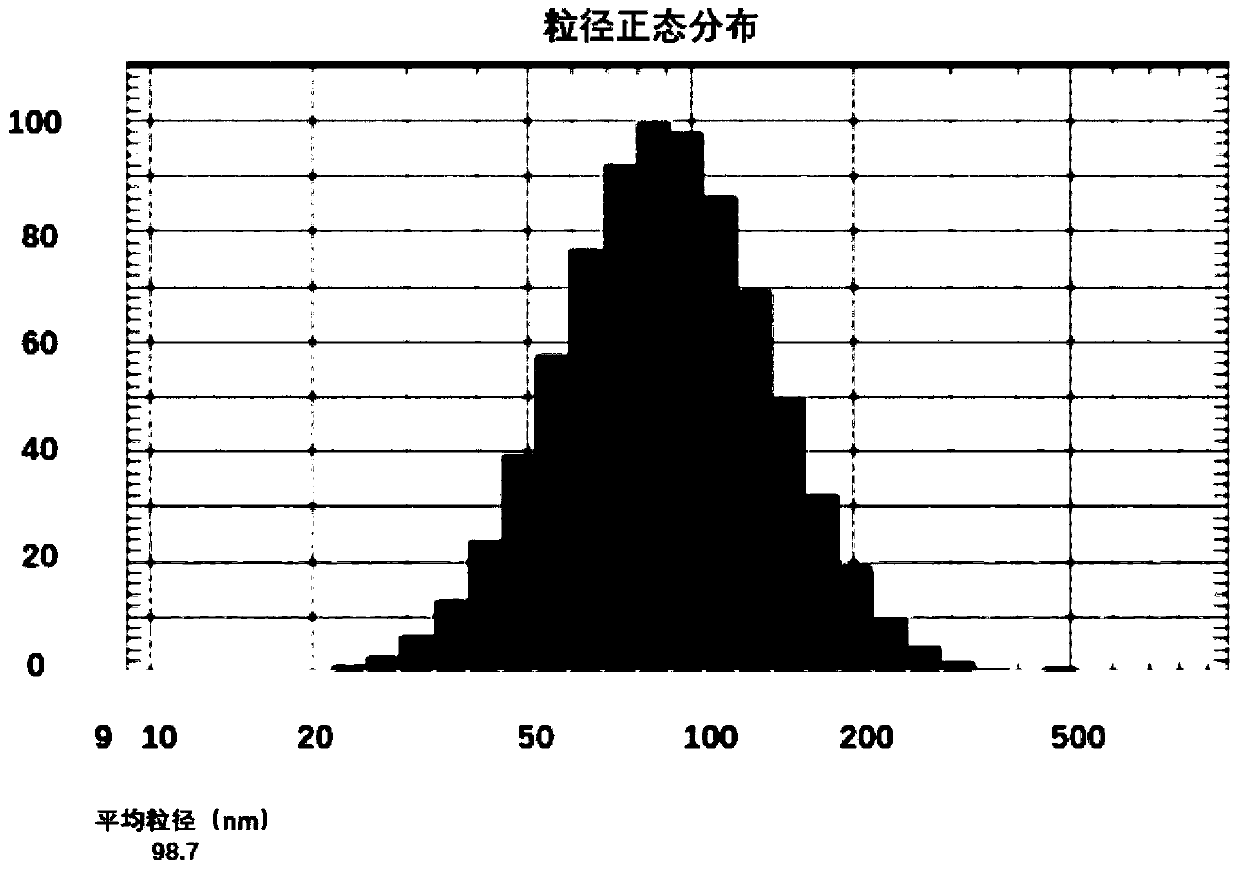
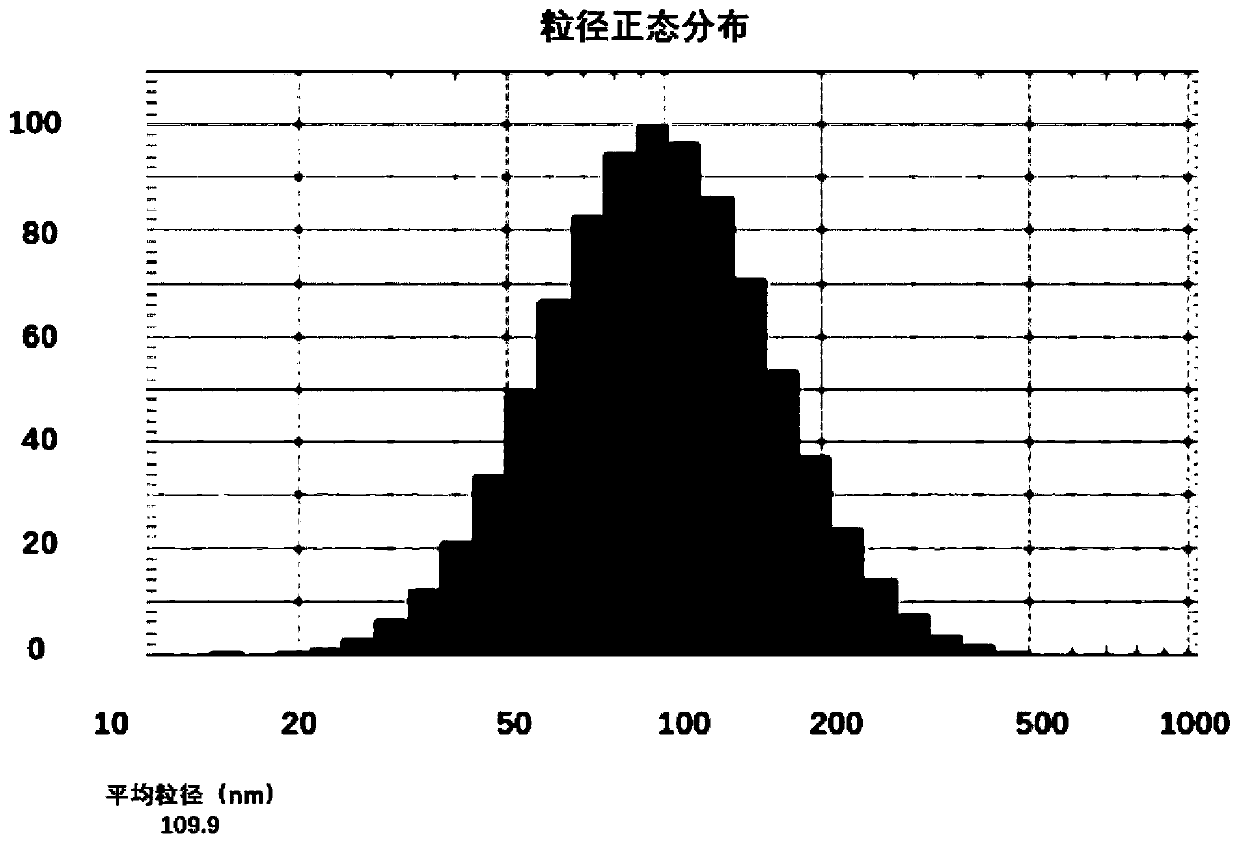
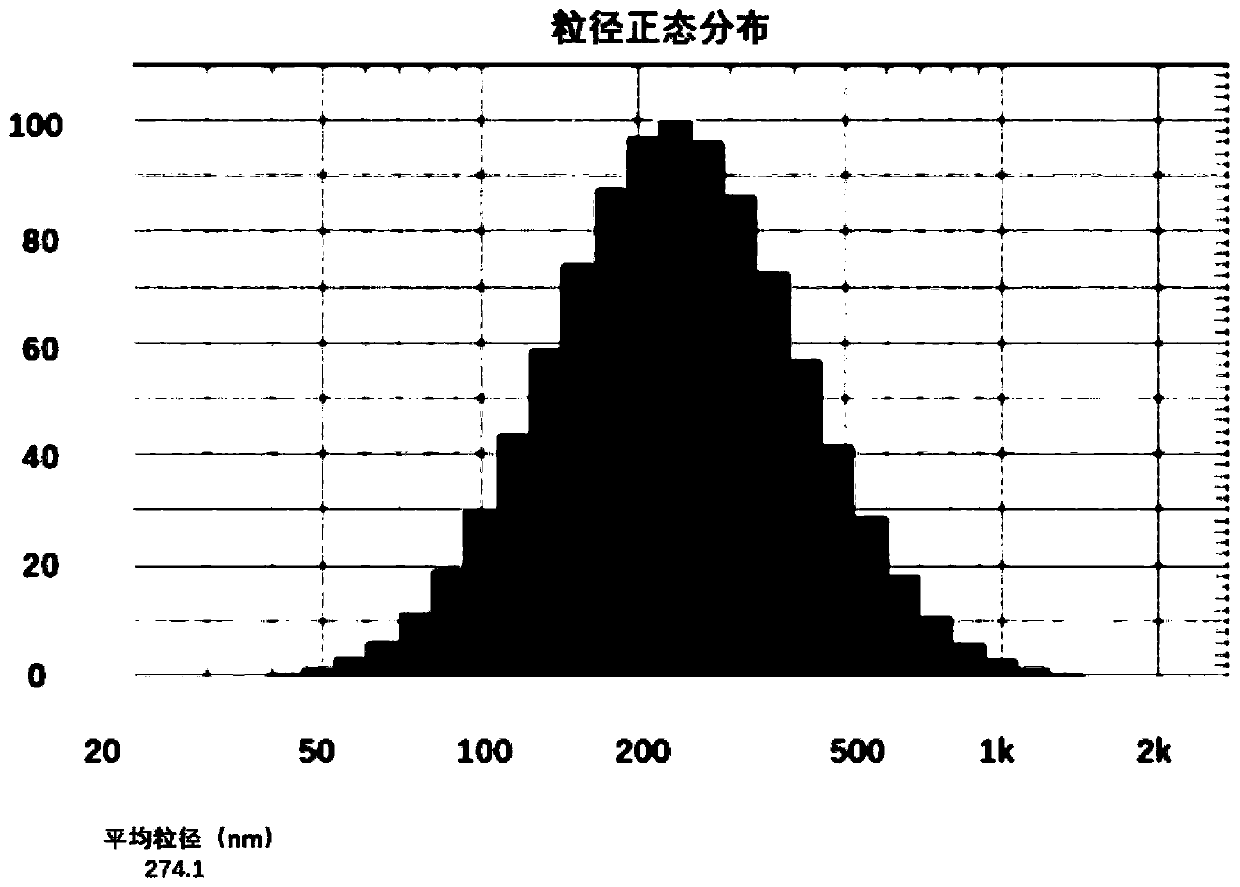
Drug composition containing abiraterone acetate and preparation method and application of drug composition
A technology of abiraterone acetate and composition, which is applied in the direction of drug combination, medical preparations containing active ingredients, medical preparations with non-active ingredients, etc., can solve the problems such as complex preparation process of compound preparations, and achieve the improvement of drug biological Utilization, increase penetrability, and meet the effect of long-term storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] The present embodiment provides a pharmaceutical composition containing abiraterone acetate, comprising the following components:
[0118]
[0119] This embodiment further provides an abiraterone acetate capsule, which uses the above-mentioned pharmaceutical composition as the content, and the content is filled in the capsule shell.
[0120] The preparation method is provided as follows:
[0121] Take hydrogenated castor oil, glycerol monooleate, and polyoxyethylene castor oil EL35, then add abiraterone acetate, sonicate for 10 minutes under dark conditions, use mechanical stirring (300rpm), stir for 20 minutes, make it fully dissolved, and then add propylene glycol And ethanol until it forms a transparent and uniform self-emulsifying solution, filled in soft capsules or sealed in hard capsules under nitrogen protection conditions for storage.
Embodiment 2
[0123] The present embodiment provides a pharmaceutical composition containing abiraterone acetate, comprising the following components:
[0124]
[0125]
[0126] This embodiment further provides an abiraterone acetate capsule, which uses the above-mentioned pharmaceutical composition as the content, and the content is filled in the capsule shell.
[0127] The preparation method is provided as follows:
[0128] Take Span 80, hydrogenated castor oil, glycerol monooleate, polyoxyethylene castor oil EL35, then add abiraterone acetate, use mechanical stirring (300rpm), stir for 25min, make it fully dissolved, then add BHA, BHT, propylene glycol And ethanol until it forms a transparent and uniform self-emulsifying solution, filled in soft capsules or sealed in hard capsules under nitrogen protection conditions for storage.
Embodiment 3
[0130] The present embodiment provides a pharmaceutical composition containing abiraterone acetate, comprising the following components:
[0131]
[0132] This embodiment further provides an abiraterone acetate capsule, which uses the above-mentioned pharmaceutical composition as the content, and the content is filled in the capsule shell.
[0133] The preparation method is provided as follows:
[0134] Take medium-chain triglycerides, glycerol monolinoleate, polyoxyethylene 40 hydrogenated castor oil, add abiraterone acetate, use mechanical stirring (300rpm), stir for 30min, make it fully dissolved, then add diethylene glycol mono Ethyl ether, BHT, and BHA are treated to form a transparent and uniform self-emulsifying solution, filled in soft capsules or sealed in hard capsules under nitrogen protection conditions for storage.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com