A class of polyfluorene derivatives containing siloxane side chains suitable for inkjet printing process and its preparation method and application
An inkjet printing technology containing siloxane, which is applied in the field of polyfluorene derivatives containing siloxane side chains and its preparation, can solve the problems of high ink viscosity, easy blockage of nozzles, and large molecular weight, and achieve low cost, The effect of simple structure and excellent photoelectric performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
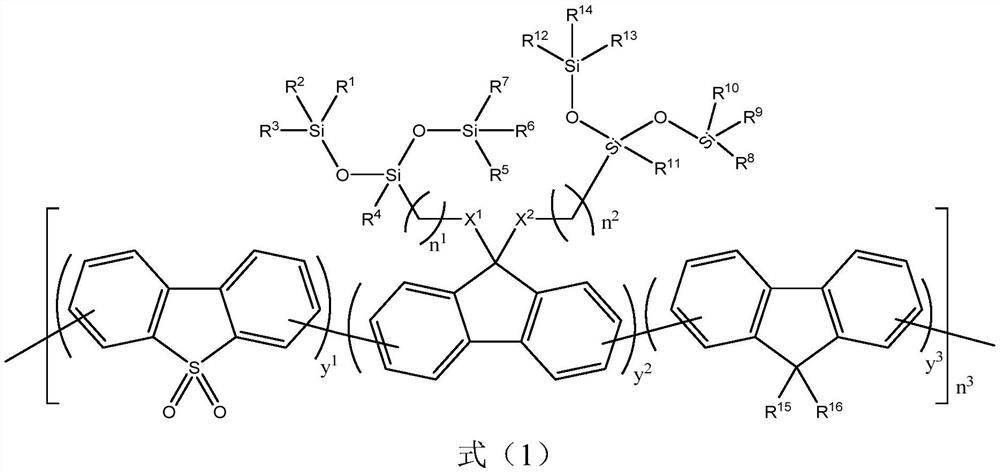
[0057] Embodiment 1: the preparation of polymer P1
[0058] (1) Preparation of compound M1: Add purchased fluorene (1.66g, 10mmol), 4-bromo-1-butene (3.24g, 24mmol), potassium hydroxide (1.41g, 25mmol) and DMSO (60ml), stirred at room temperature under nitrogen protection for 8 hours. After the reaction was completed, it was poured into 200ml of deionized water, and the organic phase was extracted three times with 100mL of chloroform, then dried by adding anhydrous magnesium sulfate, purified with a silica gel column after evaporating chloroform (eluent is n-hexane), and obtained 2.14g of a white solid. Yield 78%.
[0059] (2) Preparation of compound M2: Add compound M1 (2.74g, 10mmol) into a 200ml two-necked bottle, purchased 1,1,1,3,5,5,5-heptamethyltrisiloxane (5.34g , 24mmol), Castel catalyst (2mL) and toluene (100ml), heated to 50°C under nitrogen protection, and stirred for 24 hours. After the reaction was completed, the mixture was concentrated under vacuum, and the ...
Embodiment 2
[0067] Embodiment 2: the preparation of polymer P2
[0068] (1) Synthesis of compound M8: 2,7-dibromofluorenone (33.80g, 100mmol), phenol (28.23g, 300mmol), methanesulfonic acid (33.64g, 350mmol) and four Carbon chloride (300ml) was heated under reflux at 80°C and stirred for 24 hours under the protection of nitrogen. Suction filtration after cooling, the filter residue was washed twice with distilled water (150ml) and dichloromethane (150ml) successively, and then further purified with a silica gel column (the eluent was petroleum ether:ethyl acetate=8:1) to obtain a white solid 42.30 g, yield 83%. 1 H NMR (500MHz, DMSO) δ (ppm): 9.40 (s, 2H), 7.90 (d, J = 8.1Hz, 2H), 7.58 (dd, J = 8.1, 1.8Hz, 2H), 7.48 (d, J =1.7Hz, 2H), 6.92-6.84(m, 4H), 6.69-6.61(m, 4H).
[0069](2) Synthesis of compound M9: Add compound M8 (25.41g, 50mmol), potassium carbonate (20.7g, 150mmol) and N,N-dimethylformamide (150ml) into a 300ml two-necked bottle, under nitrogen protection, Heat to 90°C and...
Embodiment 3
[0074] Embodiment 3: the preparation of polymer light-emitting diode
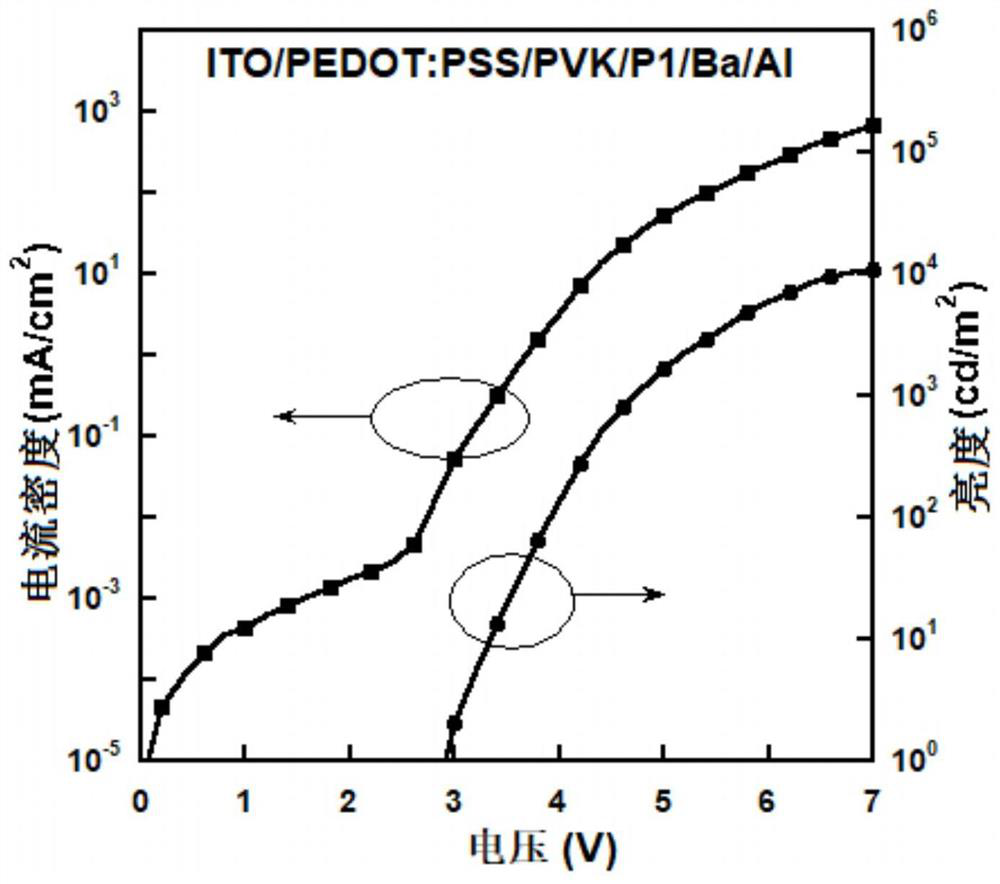
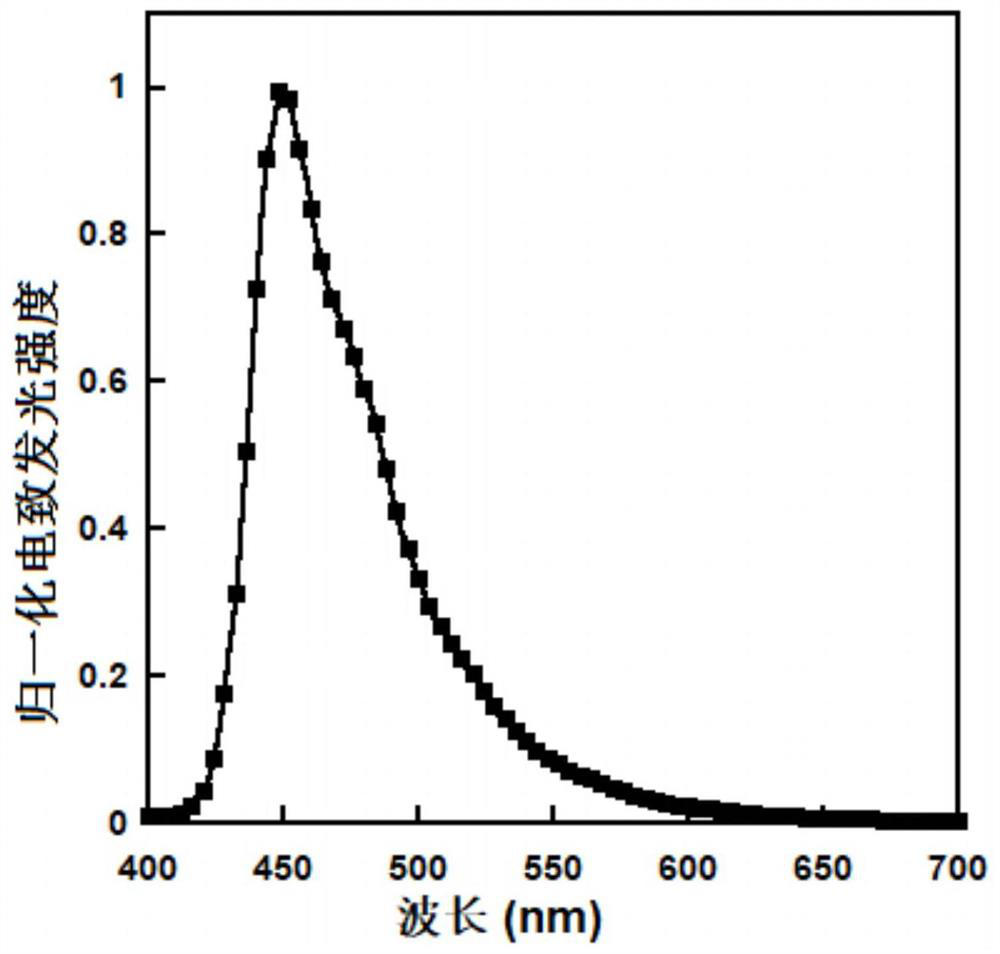
[0075] Take the pre-made indium tin oxide (ITO) glass with a sheet resistance of 10Ω, ultrasonically clean it with acetone, detergent, deionized water and isopropanol in sequence, and treat it with plasma for 1.5 minutes; spin-coat the ITO doped with polystyrene A polyethoxythiophene (PEDOT:PSS=1:6, w / w) film of ethylene sulfonic acid with a thickness of 40 nm; the PEDOT:PSS film was dried in a vacuum oven at 75°C for 12 hours; subsequently spin-coated with PEDOT: The indium tin oxide (ITO) glass of PSS was transferred into a glove box filled with nitrogen to spin-coat the hole transport layer and the light-emitting layer; the chlorobenzene solution (5mg / mL) of polyvinylcarbazole (PVK) was spin-coated on PEDOT:PSS The surface of the film has a thickness of 20nm as the hole transport layer; then the xylene solution (10mg / mL) of the polymer P0, P1 or P2 is spin-coated on the surface of the PVK film with a thi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com