Drug-loaded bacterial outer membrane vesicle,and preparation method and application thereof
A technology of outer membrane vesicles and bacteria, which can be used in antibacterial drugs, pharmaceutical formulations, microcapsules, etc., and can solve the problems of less research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
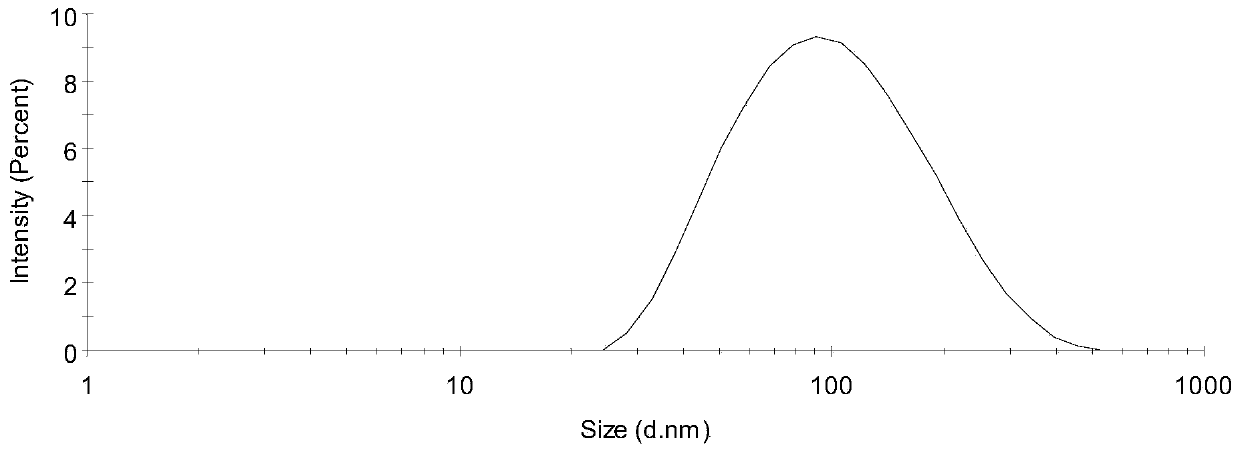
[0079] Example 1, Preparation and Characterization of Bacterial Outer Membrane Vesicles (OMV)
[0080] 1. Preparation
[0081] 1. Pseudomonas aeruginosa was inoculated on a TSA medium plate and cultured at 37°C for 24 hours.
[0082] 2. After completing step 1, use an inoculation loop to pick colonies and inoculate them into 8 mL of LB broth medium, and culture them with shaking at 37°C and 80 rpm for 24 hours.
[0083] 3. After step 2 is completed, transfer the entire culture system to 40 mL LB broth medium, and shake at 37°C and 80 rpm for 180 min.
[0084] 4. After completing step 3, transfer the entire culture system to 150 mL LB broth medium, add 60 mg D-cycloserine (membrane vesicle inducer), and culture with shaking at 37° C. and 80 rpm for 24 hours.
[0085] 5. After completing step 4, divide the culture system into centrifuge tubes, centrifuge at 10,000 g for 10 minutes, discard the precipitate, collect the supernatant, combine the supernatant, filter it with a micr...
Embodiment 2
[0094] Example 2, preparation of drug-loaded ethosome OMV / drug-loaded ordinary liposome OMV / drug-loaded OMV
[0095] 1. Preparation of OMVs
[0096] Same as 1 to 5 of step 1 of embodiment 1.
[0097] 6. After completing step 5, divide into centrifuge tubes, centrifuge at 10000g for 20min, discard the supernatant, collect the precipitate, combine the precipitate, resuspend the precipitate in sterilized PBS buffer, and then transfer to MWCO 8000-14000 In the standard dialysis bag, place the dialysis bag in PBS buffer solution for dialysis for 24 hours, collect the liquid phase in the dialysis bag, adjust the protein concentration with PBS buffer solution so that the protein concentration is 1 mg / ml, which is the bacterial outer membrane vesicle solution (also known as OMV solution). Store at 4°C.
[0098] 2. Preparation of drug-loaded ethosome OMV
[0099] A method of blending drug-loaded ethosomes and OMVs through membrane extrusion was adopted.
[0100] 1. Take a round bo...
Embodiment 3
[0166] Embodiment 3, in vitro antibacterial activity test
[0167] The test substances are respectively: the clarithromycin ethosome OMV preparation prepared in Example 2 (marked as Lip-OMV-CLA), the clarithromycin OMV preparation prepared in Example 2 (marked as OMV-CLA) and clarithromycin (marked as Free CLA).
[0168] The tested bacteria were: Staphylococcus aureus (ATCC29213), Enterococcus faecalis (ATCC29212), Escherichia coli (ATCC25922), Klebsiella pneumoniae (ATCC700603). Suspend the test bacteria with MH liquid medium to obtain the test bacteria suspension.
[0169] The molten MH solid medium is naturally cooled, and when it is about to solidify, add the test substance (the test substance provides clarithromycin, and the concentration of clarithromycin in the system is 512μg / mL-1 / 8μg / mL, 2-fold gradient) , then pour flat. The test bacteria were inoculated onto the plate by point inoculation, and the inoculation amount was 10 4 CFU / point. Incubate at 37°C for 24 h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com