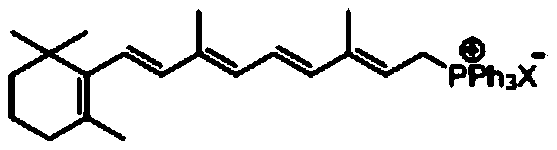
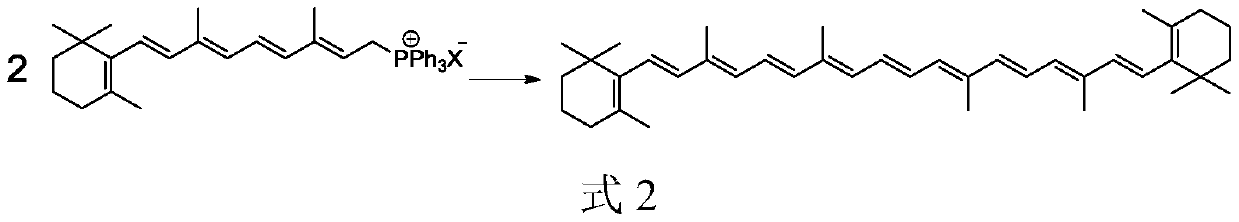
Method for preparing beta-carotene
A carotene and vitamin technology, which is applied in the field of vitamin preparation, can solve the problems of cumbersome processing process, easily oxidized products, reduced yield and the like, and achieves the effects of safe process operation, improved purity, and reduced probability of oxidative damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Preparation of Vitamin A Triphenylphosphine Salt
[0061] Add 49g of 98% pure vitamin A acetate (2.8 million IU, 0.147mol), 40.5g of triphenylphosphine (0.154mol) and 300g of methanol into a 1L three-necked flask, and cool to 0℃ in an ice-water bath with stirring. Slowly add 15.8g of concentrated sulfuric acid (0.16mol) below 5°C for about 0.5h, then continue to keep the temperature and stir for 10h, the reaction liquid becomes orange transparent liquid. Add 150g of deionized water, extract with n-hexane (100g*3 times), the lower layer is the reaction solution containing vitamin A triphenylphosphine salt (VA triphenylphosphine salt methanol-water solution), and its mass percentage composition Vitamin A triphenyl phosphine salt 15.8%, methanol 54.0%, water 27.0%, unidentified impurities 3.2%; raw material vitamin A acetate conversion rate is above 99%.
Embodiment 2
[0062] Example 2: Preparation of Vitamin A Triphenylphosphine Salt
[0063] Add 49g of 98% pure vitamin A acetate (2.8 million IU, 0.147mol), 53.9g of triphenylphosphine (0.20mol) and 400g of ethanol into a 1L three-necked flask, and cool to 0°C in an ice-water bath with stirring. Slowly drop 23.0g of concentrated sulfuric acid (0.23mol) below 5°C for about 0.5h, then continue to keep the temperature and stir for 10h, the reaction liquid becomes orange transparent liquid. Add 400g of deionized water and extract with n-hexane (100g*3 times). The lower layer is the reaction solution containing vitamin A triphenylphosphine salt (VA triphenylphosphine salt in ethanol-water solution), and its mass percentage composition Vitamin A triphenylphosphine salt 12.2%, ethanol 55.1%, water 27.6%, unidentified impurities 5.1%; vitamin A acetate conversion rate is above 99%.
Embodiment 3
[0064] Example 3: Preparation of Vitamin A Triphenylphosphine Salt
[0065] In a 1L three-necked flask, 110g VA crystallization mother liquor (in which all-trans VA acetate 42% (0.147mol); 13-cis VA acetate 38%, trans VA alcohol 14%), 46.2g triphenylphosphine (0.17mol) and 400g methanol, cooled to 0°C in an ice-water bath with stirring, slowly dripped 21.6g concentrated sulfuric acid (0.22mol) while maintaining below 5°C, dripping for about 0.5h, and then kept holding and stirring for 10h, the reaction solution became Orange transparent liquid. Add 250g of deionized water, extract with n-hexane (100g*3 times), the lower layer is the reaction solution containing vitamin A triphenylphosphine salt (VA triphenylphosphine salt methanol-water solution), and its mass percentage composition Vitamin A triphenylphosphine salt 10.6%, methanol 48.4%, water 30.2%, impurities 10.8%; the conversion rate of all-trans VA acetate in the vitamin A crystallization mother liquor is above 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com