Joint detection kit and method of influenza A virus H8N7 and H13N6
A technology of influenza A virus and H8N7, which is applied in the field of detection methods and detection kits of influenza A virus H13N6
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
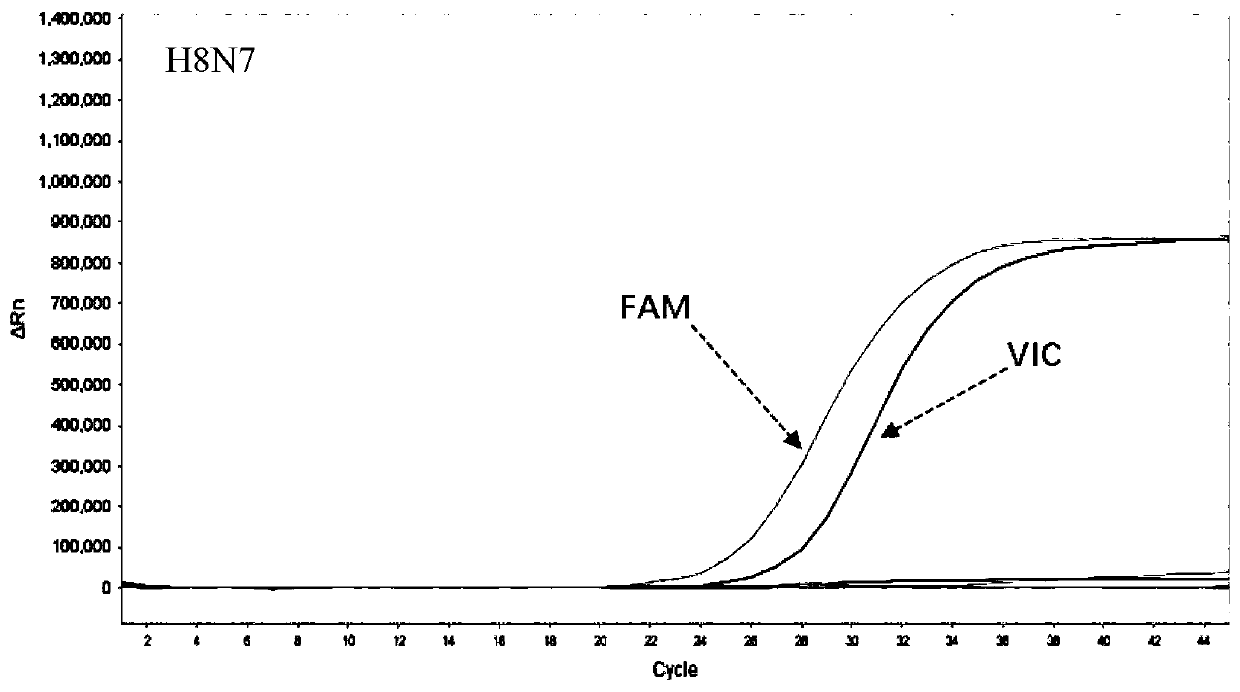
[0090] Example 1—Combined Detection Kit for Influenza A Virus H8N7 and H13N6
[0091] In this example, a kit for the dual combined detection of influenza A virus H8N7 and H13N6 by real-time fluorescent PCR, including: virus nucleic acid rapid extraction reagent, PCR amplification reagent, PCR enzyme mixture, H8N7 detection reagent, H13N6 detection reagent, positive control substance , Negative control substance. in:
[0092] (1) The quick extraction reagent of viral nucleic acid comprises lysis reagent, and lysis reagent comprises: 500mM Tris-HCl (pH10.0), 10%v / v TritonX100, 4M guanidine isothiocyanate, 100mM KCl, 0.2M EDTA (pH8 .0).
[0093] (2) The PCR amplification reagent includes a PCR amplification buffer; comprising: 2×PCRbuffer, 50mM MgCl 2 , 100 mM Tris-HCl (pH 10.0), 0.5 mM dNTPs, 5% g / ml BSA.
[0094] (3) The PCR enzyme mixture includes 0.5U / μl reverse transcriptase, 5U / μl DNA polymerase, 5×RNase inhibitor;
[0095] (4) The H8N7 detection reagent includes H8N7-...
Embodiment 2
[0100] Embodiment 2——A joint detection method of influenza A virus H8N7 and H13N6
[0101]The real-time fluorescent PCR detection method of influenza A virus H8N7 and H13N6 of this embodiment comprises the following experimental steps:
[0102] (1) Main reagents and instruments: the kit reagents in Example 1 were used; the fluorescent quantitative PCR instrument was ABI7500.
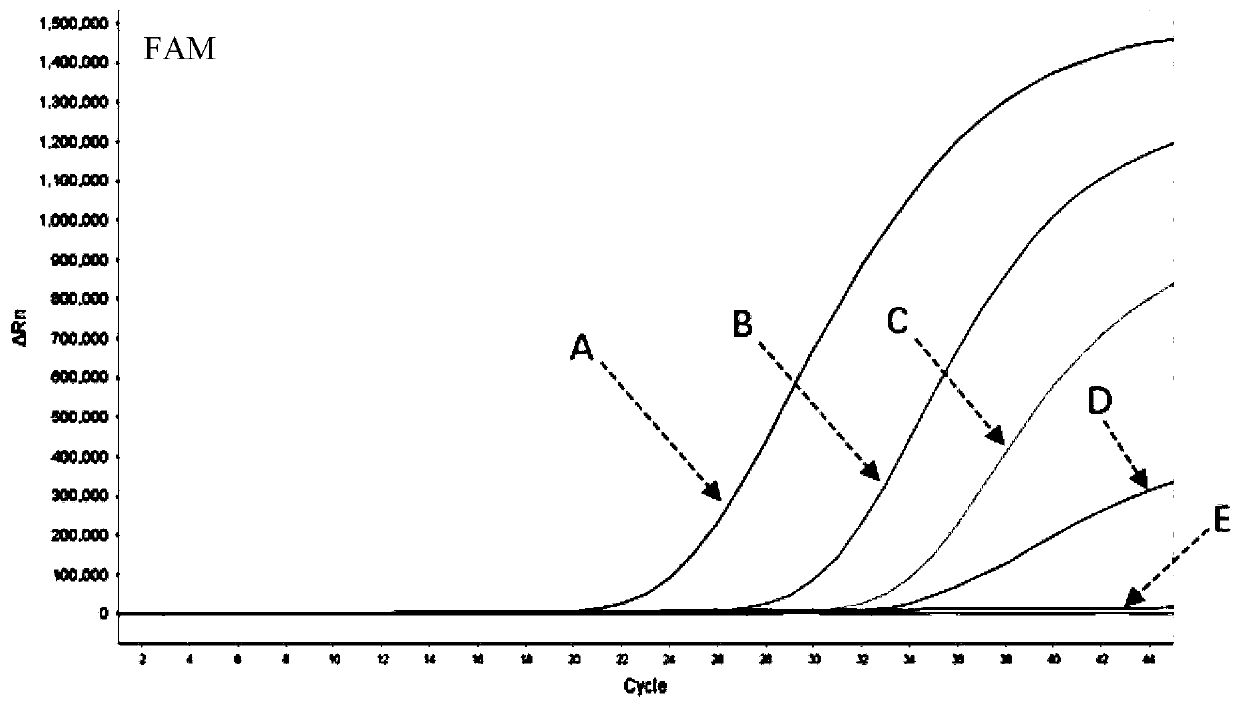
[0103] (2) Specimen preparation: Positive samples are inactivated virus after titration of human influenza A virus H8N7 and inactivated virus of human infection of influenza A virus H13N6 after titration, and then diluted with DEPC water in different times, and negative control samples are healthy people saliva swab.
[0104] (3) RNA extraction: Mix equal volumes of the viral nucleic acid rapid extraction reagent and the sample to be tested, let stand at room temperature for 5-10 minutes, and then directly use the lysed mixture for PCR detection. (4) Amplification of the target gene:
[0105] a. Desig...
Embodiment 3
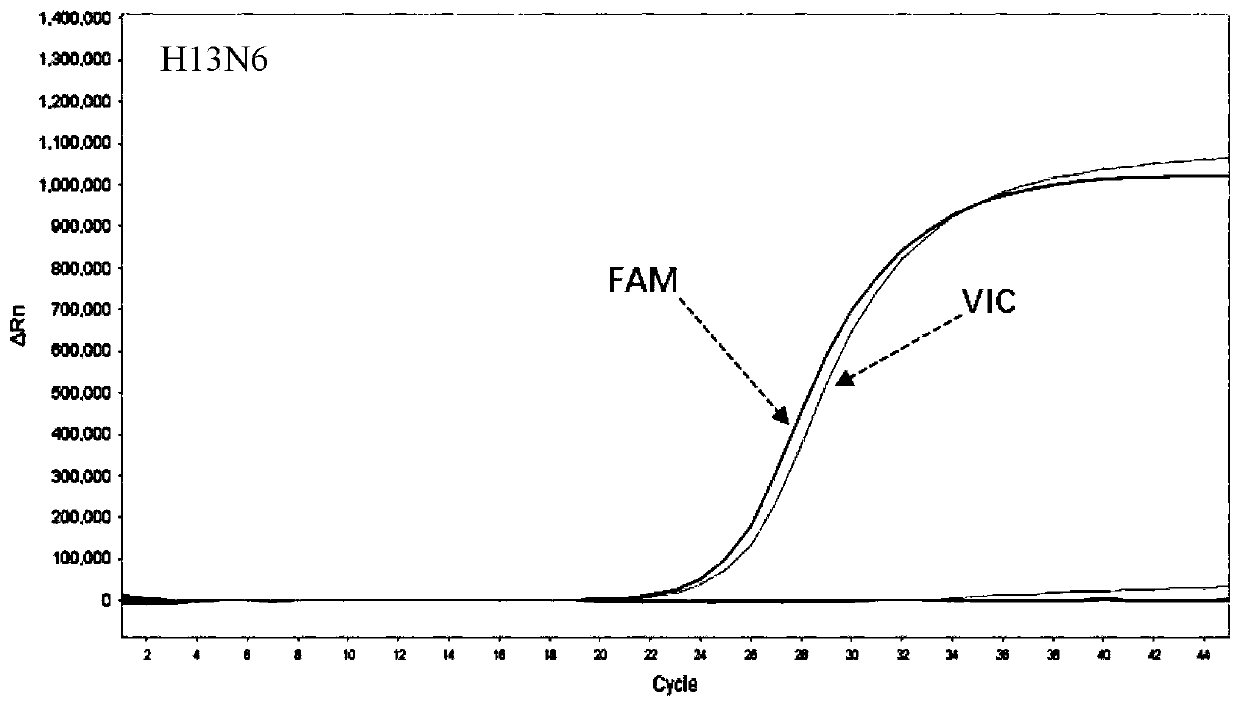
[0144] Embodiment 3——The detection kit of influenza A virus H13N6
[0145]In this embodiment, the kit for detecting influenza A virus H13N6 by real-time fluorescent PCR includes: virus nucleic acid rapid extraction reagent, PCR amplification reagent, PCR enzyme mixture, H13N6 detection reagent, positive control substance, and negative control substance. in:
[0146] (1) The viral nucleic acid rapid extraction reagent includes a lysis reagent, specifically comprising: 500mM Tris-HCl (pH10.0), 10% v / v TritonX100, 4M guanidine isothiocyanate, 100mM KCl, 0.2M EDTA (pH8. 0).
[0147] (2) The PCR amplification reagent includes a PCR amplification buffer; comprising: 2×PCRbuffer, 50mM MgCl 2 , 100 mM Tris-HCl (pH 10.0), 0.5 mM dNTPs, 5% g / ml BSA.
[0148] (3) The PCR enzyme mixture includes 0.5U / μl reverse transcriptase, 5U / μl DNA polymerase, 5×RNase inhibitor;
[0149] (4) The H13N6 detection reagent includes H13N6 specific primers and probes; H13N6-F1, H13N6-F2, H13N6-R1, H13N6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com