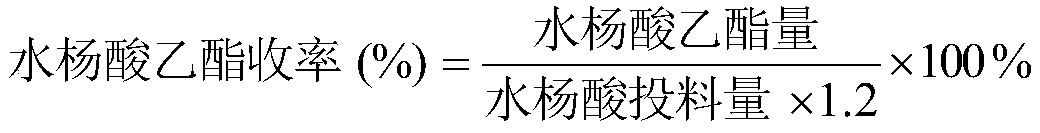Synthetic method of ethyl salicylate
A technology of ethyl salicylate and a synthesis method, which is applied in the chemical industry, can solve the problems of failing to meet the needs of industrial reactions, easily introducing other impurities, and low yields, achieving short synthesis and product purification steps, and overcoming product yields Not high, the effect of high reaction product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
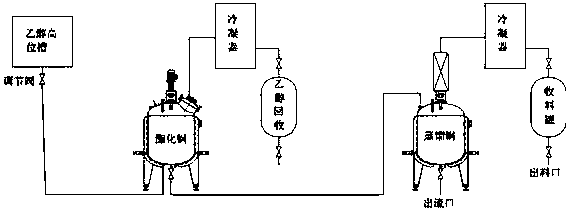
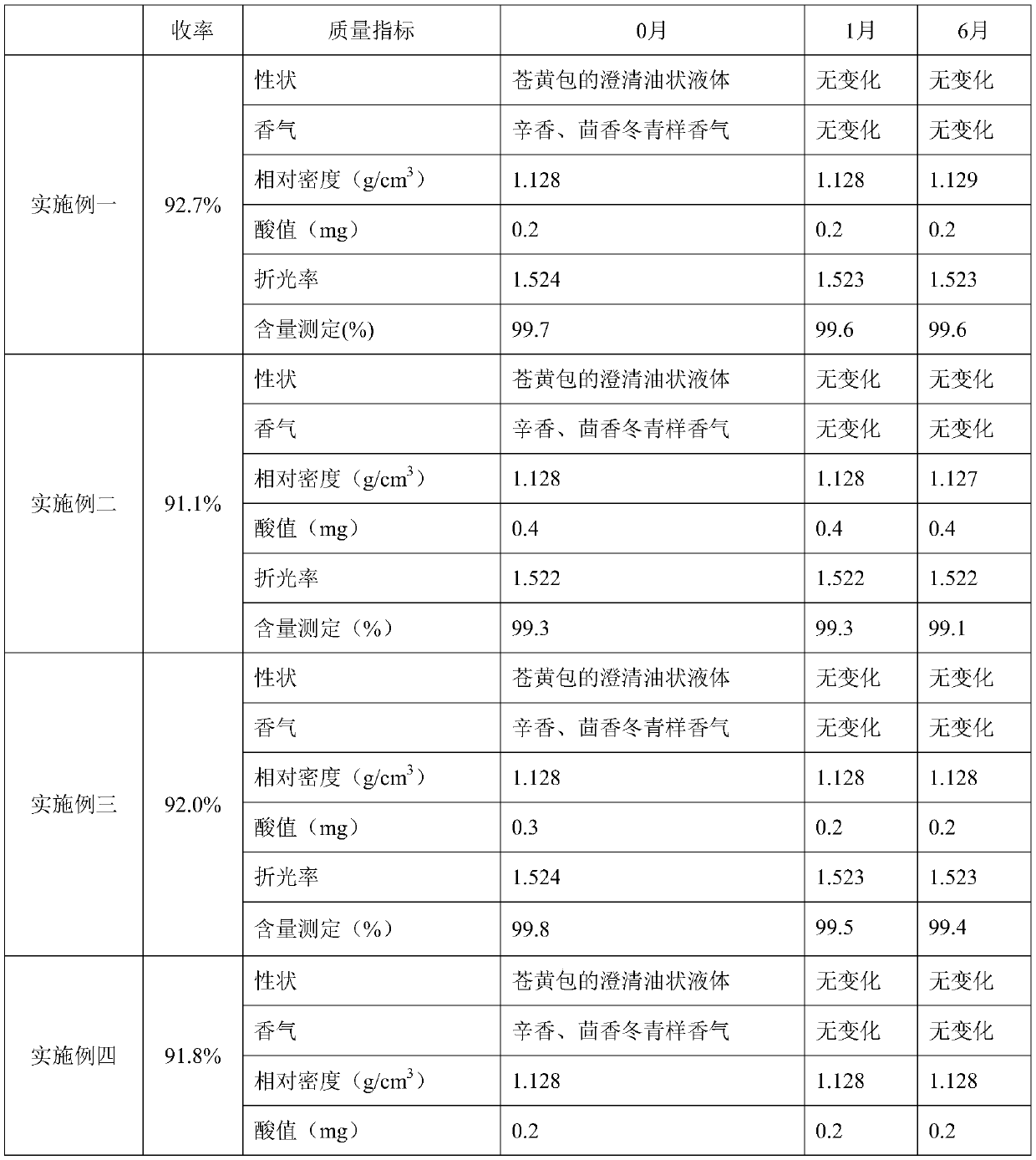
[0048] Synthesis: Put 15.0kg of 95% ethanol, 1.0kg of sulfuric acid and 50.0kg of salicylic acid into a glass-lined reaction tank in sequence, heat and reflux until about 50% or more (approximate range is enough), stop the reflux when the salicylic acid dissolves, and start distillation and collection ethanol in water. When the temperature of the reaction system rose to 110°C, add 95% ethanol to the reaction system at a rate of 2.9L / h (every 1kg of salicylic acid added ethanol 0.058L), and the temperature control of the reaction system was about 110°C. ℃, and the ethanol recovery ratio is 60%. Judge the remaining amount of salicylic acid in the reaction system, stop adding ethanol if the requirement is met, and continue to collect ethanol distilled between 100-120°C. The volume of the glass-lined reaction tank is 50L.
[0049] The method for judging the residual amount of salicylic acid in the reaction system is: during the esterification reaction, detect the concentration o...
Embodiment 2
[0053] Synthesis: Put 5.0kg of 95% ethanol, 1.0kg of sulfuric acid and 30.0kg of salicylic acid into a glass-lined reaction tank in turn, heat and reflux until about 50% of the salicylic acid dissolves, stop the reflux, and start distillation to collect the ethanol aqueous solution. When the temperature of the reaction system rose to 105° C., add 95% ethanol to the reaction system at a rate of 5.1 L / h (every 1 kg of salicylic acid added ethanol 0.17 L), and the temperature control of the reaction system was about 100 ℃, and the ethanol recovery ratio was 56%. Judge the remaining amount of salicylic acid in the reaction system, stop adding ethanol if the requirement is met, and continue to collect ethanol distilled between 100-120°C.
[0054] The method for judging the residual amount of salicylic acid in the reaction system is: detect the concentration of the low-concentration ethanol recovered during the esterification reaction, and when the ethanol concentration is greater t...
Embodiment 3
[0058] Synthesis: Put 40.0kg of 90% ethanol, 5.0kg of sulfuric acid and 50.0kg of salicylic acid into a glass-lined reaction tank in sequence, heat and reflux until about 50% or more of the salicylic acid dissolves, stop the reflux, and start distillation to collect the aqueous ethanol solution. When the temperature of the reaction system rises to 110°C, add 90% ethanol to the reaction system at a rate of 9.75L / h ((per hour, every 1kg of salicylic acid adds ethanol 0.195L)), and the temperature of the reaction system is controlled by about It was 118° C., and the ethanol recovery ratio was 63%. Judge the remaining amount of salicylic acid in the reaction system, stop adding ethanol if the requirement is met, and continue to collect ethanol distilled between 100-120°C.
[0059] The method for judging the residual amount of salicylic acid in the reaction system is: detect the concentration of the low-concentration ethanol recovered during the esterification reaction, and when th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com