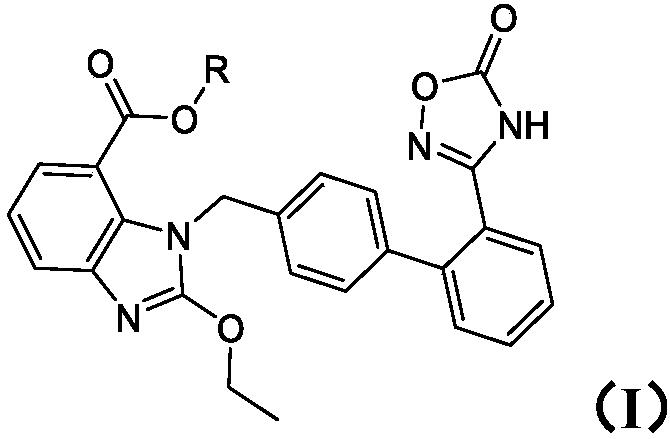
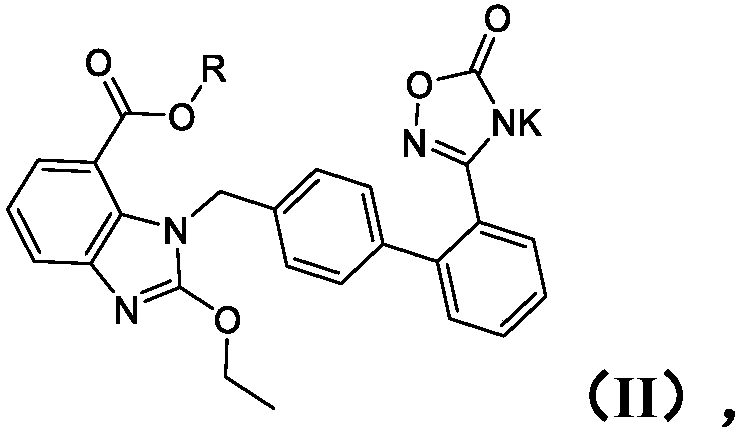
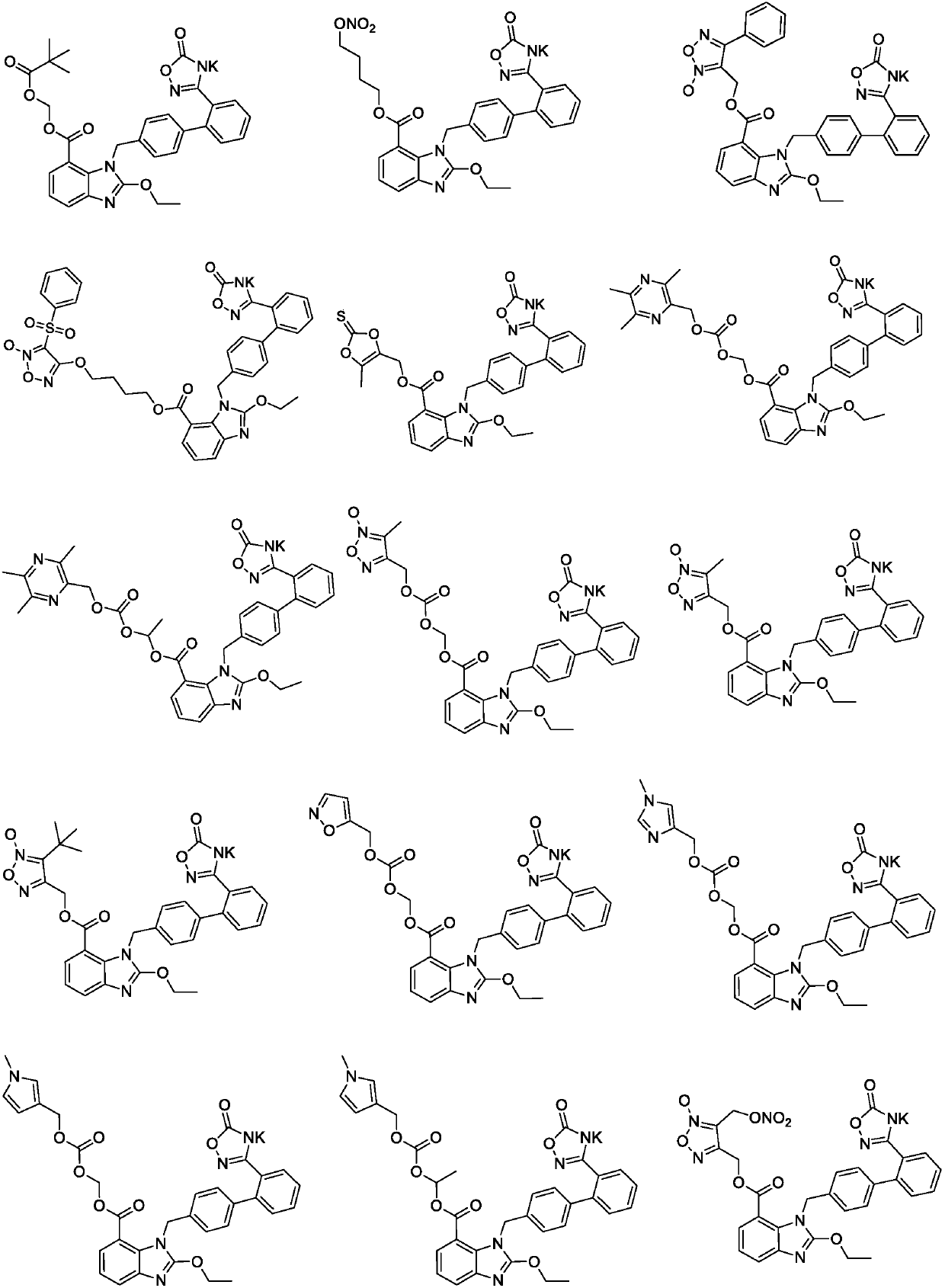
Preparation method of medical composition
A composition and drug technology, applied in the field of medicine, can solve the problems of good pharmaceutical composition products, difficult to obtain dissolution and release properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Embodiment 1 prepares the first active component
[0152] Add Shakubiqu into acetone solvent, stir at room temperature, cool to 0-10°C, add a little excess of concentrated ammonia water dropwise, after the dropwise addition, continue stirring for 4 hours, filter, wash with acetone, and dry in vacuo to obtain AHU377 ammonium salt with a purity of Greater than 99.5%, MS: m / z=412.3 (M+H) + .
[0153] The preparation of AHU 377K is similar to this process, replacing concentrated ammonia with potassium hydroxide.
Embodiment 2
[0154] Embodiment 2 prepares the second active component: the preparation of compound 1K
[0155]
[0156] Dissolve compound 1 (1.0g) in dichloromethane (5ml), stir at room temperature to form a solution, add potassium phthalimide (0.27g) to the solution, keep warm for 4h, cool to -50°C, filter , and the solvent was spin-dried to obtain a solid compound 1K (amorphous).
[0157] Melting point: 135-145°C.
[0158] MS / HRMS m / z: 717[M+H] + ;677[M-K] - .
[0159] 1 H-NMR (400MHz, DMSO-d 6 )δ: 1.44(t, 3H), 1.46(t, 3H), 2.38(s, 3H), 2.41(s, 3H), 2.44(s, 3H), 4.64(q, 2H), 5.29(d, 1H ), 5.32(d, 1H), 5.52(d, 1H), 5.56(d, 1H), 6.86(q, 1H), 6.90(d, 2H), 7.18(m, 2H), 7.22(d, 2H) , 7.33 (m, 1H), 7.36 (m, 1H), 7.46 (d, 1H), 7.52 (dd, 1H), 7.75 (d, 1H).
[0160] The study found that: the composition (excluding preparation auxiliary materials) consisting only of the products of Examples 1 and 2 (100mg: 50mg), at a relative humidity of 92.5% and a temperature of 25°C, the moisture abso...
Embodiment 3
[0162]
[0163]
[0164] Weigh the compound 1K, AHU 377 ammonium salt, mannitol, microcrystalline cellulose, colloidal silicon dioxide, and crospovidone in the first auxiliary material, mix them through a 40-mesh sieve and put them into the mixer, and mix them for 6 minutes. Then add the first auxiliary material magnesium stearate in the added part, and mix for 3 minutes.
[0165] A dry granulator was used to prepare granules, and the specific granulation conditions were as follows: the roll width was 25mm, the roll diameter was 200mm, and the roll gap was 2mm; the feeding speed was 34rpm, the pressing wheel speed was 3rpm, and the granulation speed was 550rpm.
[0166] Take by weighing the colloidal silicon dioxide in the second auxiliary material of recipe quantity, after crossing 40 mesh sieves, mix with crospovidone, magnesium stearate (additional part) and the above-mentioned granules in the second auxiliary material, drop into mixer and mix for 3 Minutes, a blend o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com