Novel synthesis method of alkoxymethylamine compound
A technology of alkoxymethylamines and compounds, which is applied in the field of preparation of alkoxymethylamines, can solve the problems of non-recyclable use, large discharge of three wastes, large amount of hydrochloric acid, etc., and achieve environmental protection and increase in reaction yield , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
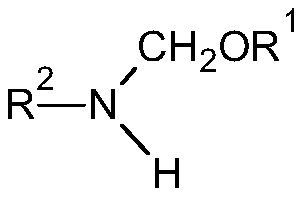
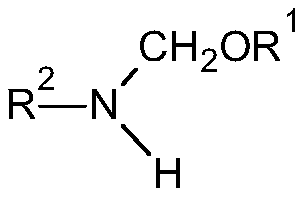
[0039] Preparation of 2,6-diethyl-N-(methoxymethyl)aniline
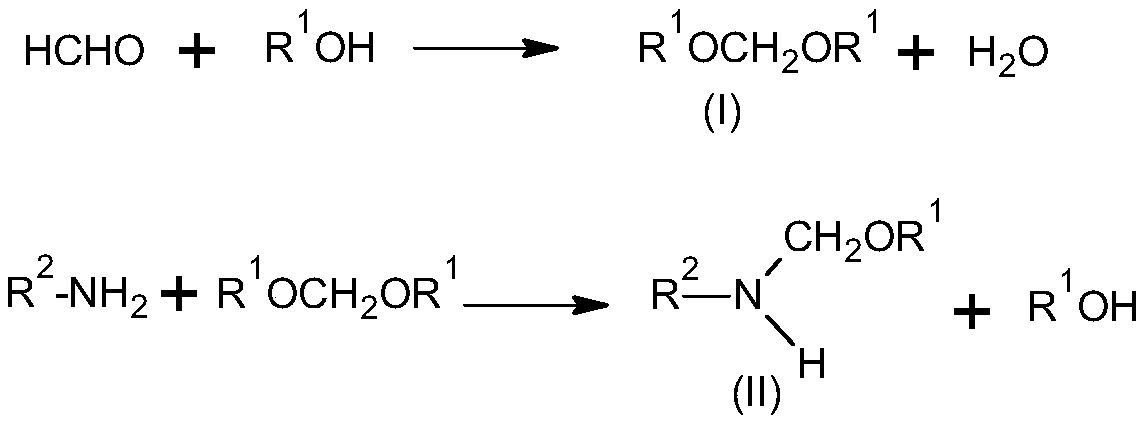
[0040] According to the feed ratio in Table 1, add formaldehyde and methanol into a three-necked flask equipped with a condenser and a thermometer, add a catalyst, heat up to reflux, and separate the generated water during the reaction. After HPLC detected that the reaction was complete, dimethoxymethane intermediate was obtained by distillation under reduced pressure.
[0041] Feed ratio and reaction conditions of table 1 acetal reaction
[0042]
[0043] According to the proportioning and reaction conditions of Table 2, the dimethoxymethane intermediate (A), 2,6-diethylaniline (B) and solvent prepared in Example 1-5 were added to the reaction flask and kept warm Reaction, HPLC detection After the completion of the reaction, separate the generated methanol and solvent recovery, and distill to obtain 2,6-diethyl-N-(methoxymethyl)aniline.
[0044] Feed ratio and reaction conditions of table 2 alkylation reaction ...
Embodiment 6
[0047] Example 6 2,6-diethyl-N-(ethoxymethyl)benzene
[0048] Add 1 mol of formaldehyde and 2.05 mol of ethanol into a three-necked flask equipped with a thermometer, add 0.86 g of p-toluenesulfonic acid, heat up to reflux, and separate the generated water during the reaction. After completion of the reaction as detected by HPLC, diethoxymethane was obtained by vacuum distillation with a yield of 96% and a content of 98.8%.
[0049] Add 0.3mol of diethoxymethane, 0.5mol of 2,6-methylethylaniline and 100mL of benzene into the reaction flask, keep it warm at 75-80°C for 15 hours, and separate out the ethanol and benzene generated after the reaction is completed by HPLC. 2,6-diethyl-N-(ethoxymethyl)aniline was obtained with a yield of 96.2% and a content of 98.3%.
[0050] The total yield of the above two-step reaction is 92.35%.
Embodiment 7
[0051] Example 7 2,6-diethyl-N-(butoxymethyl)aniline
[0052]Add 1 mol of formaldehyde and 2.05 mol of butanol into a three-necked flask equipped with a thermometer, add 0.44 g of sulfamic acid, heat up to reflux, and separate the generated water during the reaction. After the reaction was complete as detected by HPLC, dibutoxymethane was obtained by distillation under reduced pressure, with a yield of 96.8% and a content of 98.5%.
[0053] Add 0.55 mol of dibutoxymethane, 0.5 mol of 2,6-diethylaniline and 100 mL of xylene into the reaction flask, keep warm at 135-140°C for 9 hours, and separate the produced butanol and xylene for recovery after HPLC detection. Apply mechanically, and distill to obtain 2,6-diethyl-N-(butoxymethyl)aniline with a yield of 96.2% and a content of 98.3%.
[0054] The total yield of the above two-step reaction is 93.12%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com