5-aminolevulinic acid synthetase mutant and its host cell and application
A technology of aminolevulinic acid and host cells, which is applied to mutants of 5-aminolevulinic acid synthase, host cells and application fields thereof, and can solve the problems of few and low expression activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1. Construction of ALA synthetase mutation carrier
[0095] Utilize the Stratagene Series XL-II site-directed mutagenesis kit, designed 19 pairs of primers (see Table 2), using the pEC-XK99E-hemA wild-type plasmid as a template (the construction process refers to the construction and fermentation of the pathway for the synthesis of 5-aminolevulinic acid by Corynebacterium glutamicum Optimization [J]. Biotechnology Bulletin, 2017,33(01):148-156.), using the above primers for PCR amplification, the 11th amino acid residue of HemA was mutated into alanine (A), sperm amino acid (R), cysteine (C), isoleucine (I), methionine (M), serine (S), threonine (T), valine (V). The PCR reaction conditions are: 95°C for 5min, 10 cycles (95°C for 30s, 74°C-65°C for 30s, 72°C for 4.5min), 13 cycles (95°C for 30s, 65°C for 30s, 72°C for 4.5min), 72°C 10min. PCR amplification system (50 μL): template 1 μL, upstream and downstream primers 1 μL, dNTP mix 4 μL, 5× Fast Pfu F...
Embodiment 2
[0098] Example 2. Recombinant strain construction and fermentation verification
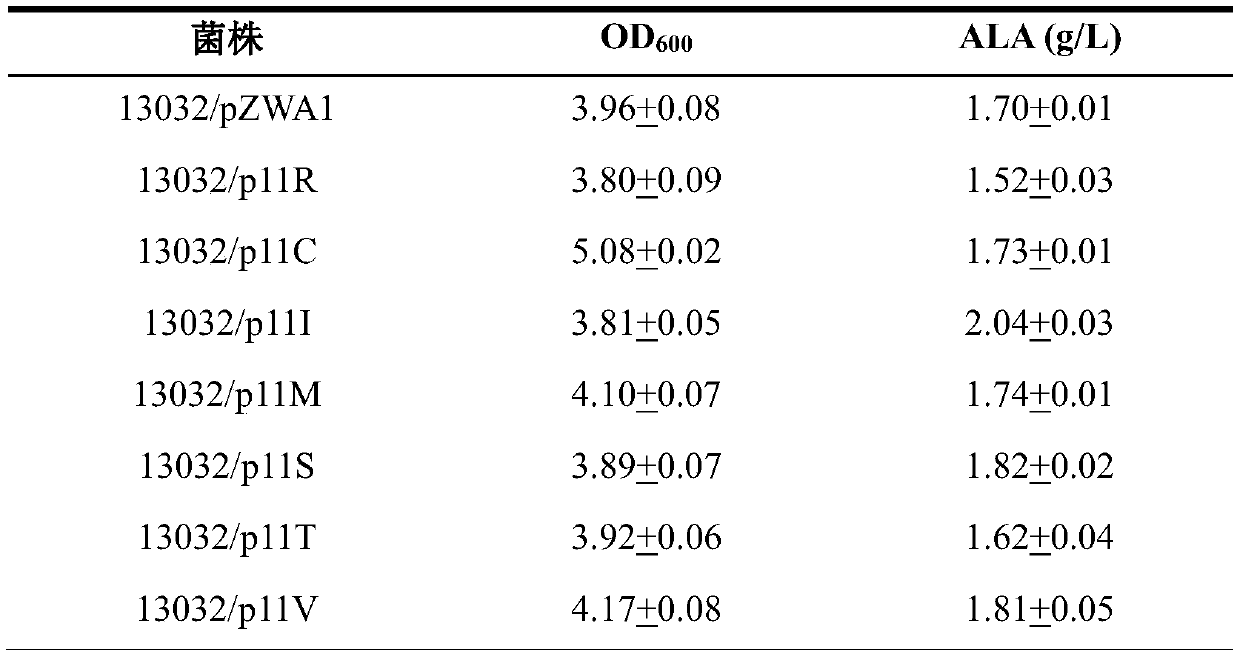
[0099] Transform the expression vector of the correct ALA synthetase mutation into C. glutamicum ATCC13032 strain to obtain the engineering strain, carry out 24-well plate ALA fermentation on the ALA synthase mutant engineering strain, and the mutant engineering strain is in the M9 medium containing yeast powder. Cultured in 24-well plate for 16h as seed solution, according to the initial OD 600Transfer 0.5 to a 24-well plate containing M9 medium for fermentation verification, culture for 3 hours, add the inducer IPTG with a final concentration of 100uM, and finish the fermentation after 24 hours, and measure the crude enzyme activity of ALA synthase and ALA production. The detection methods of crude enzyme activity, ALA detection and glucose analysis are as described in the "Materials and Methods" section. After testing, compared with the wild-type strain, the crude enzyme activity of each muta...
Embodiment 35
[0103] Example 3.5L fermenter verification
[0104] Select the mutant (p11I) expressing bacterial strain with the best above-mentioned effect to carry out 5L fermenter verification, and fermentation medium composition is: (NH 4 ) 2 SO 4 5g / L, KH 2 PO 4 5g / L, corn steep liquor dry powder 2g / L, MgSO 4 7H 2 O 2g / L, thiamine hydrochloride 1mg / L, fermentation process control temperature 30°C, pH 6.5, dissolved oxygen not less than 30%. The results showed that the ALA production of the above strains reached 20g / L respectively, which was 16.2% higher than that of the control strain (17.2g / L), indicating that the engineering strains expressing mutants could also effectively improve the production of ALA at the fermenter level.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com