Application of dna carbon dot-silicon nano hydrogel material in the preparation of reagents for fluorescence detection of carcinoembryonic antigen in serum
A technology for fluorescence detection and carcinoembryonic antigen, which is applied in the application field of reagents, can solve the problems of poor stability, long construction time, and poor accuracy, and achieve good stability, short synthesis time, and reduce the cost of material construction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
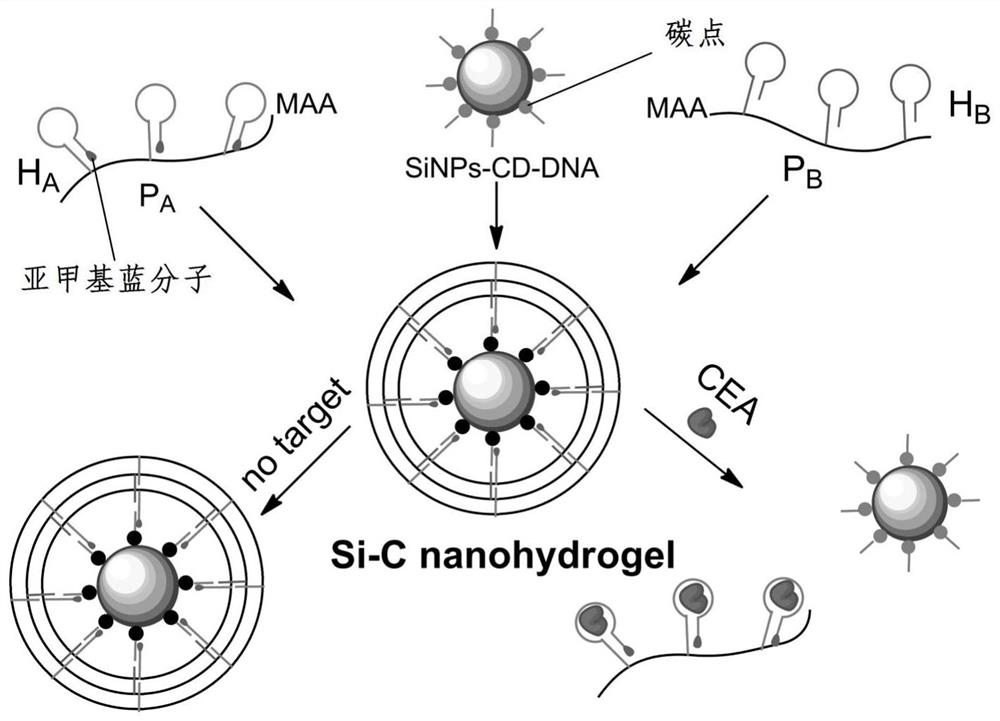
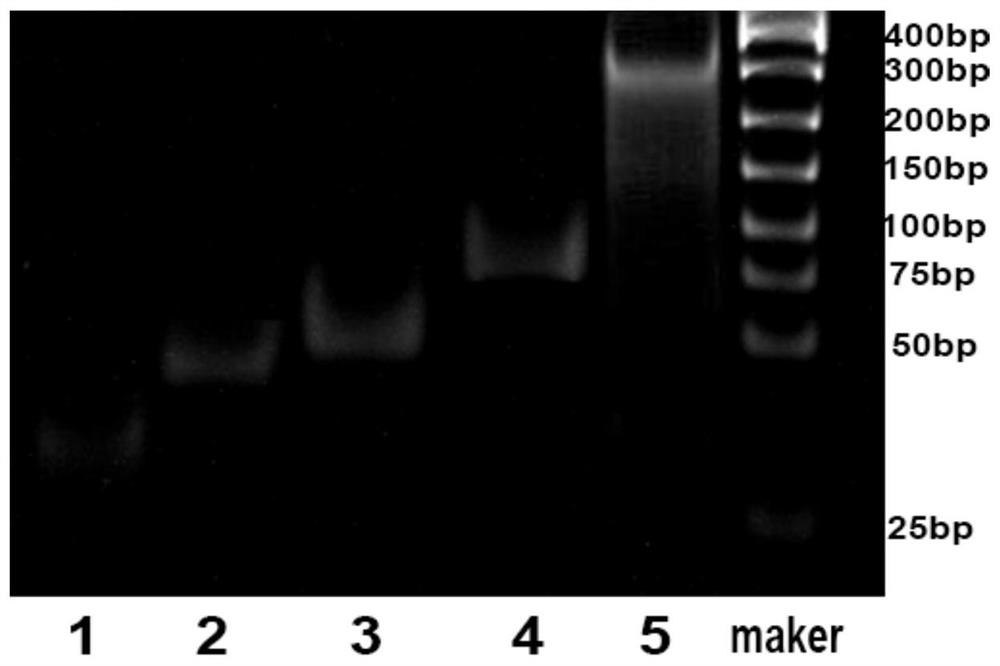
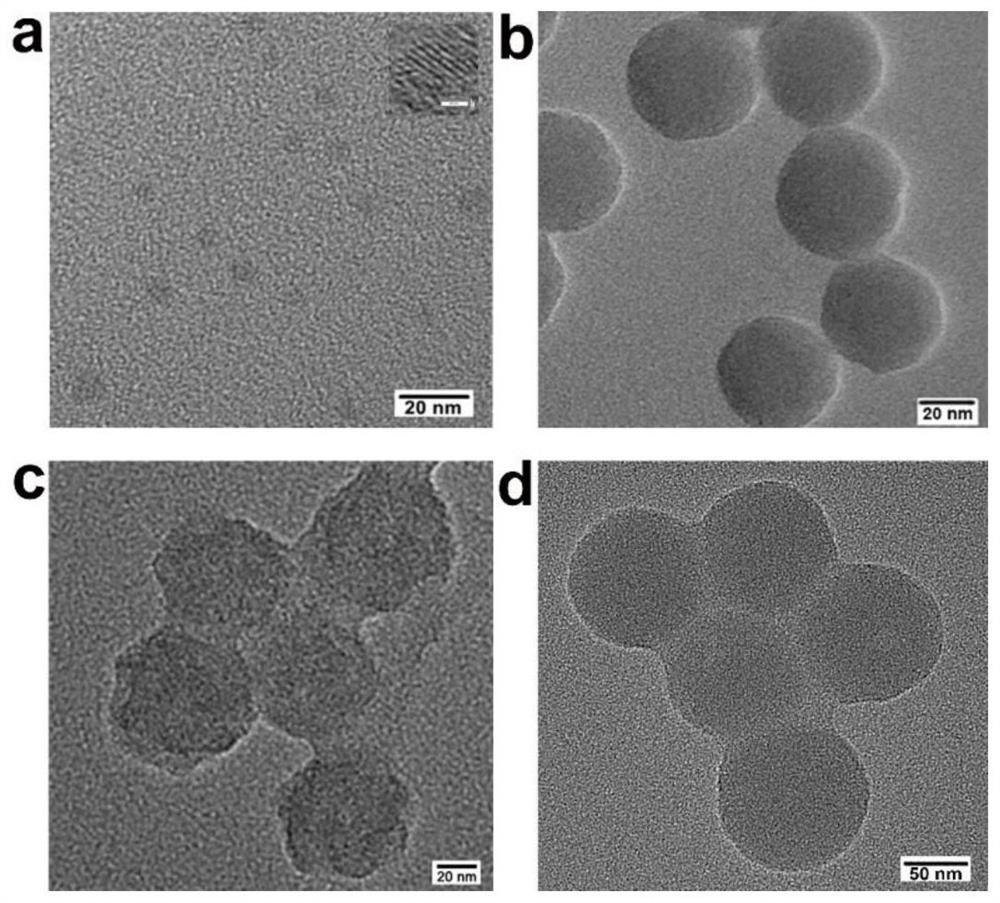
[0044] Example 1: Preparation of DNA carbon dots-silicon nanohydrogels
[0045] (1) Preparation of fluorescent carbon dots
[0046] S1. Weigh 0.6g of urea and 0.3g of citric acid in a beaker, add 10mL of deionized water to dissolve the powder completely, heat it in a microwave at 800W for 4min, and put it in a vacuum drying oven at 60℃ for 1h. Weigh the dry powder mass. Put the black powder into 40 mL of deionized water, centrifuge at 3000 rpm for 20 min, remove the filter residue, and take the supernatant, that is, the carbon dot stock solution.
[0047] (2) Preparation of SiNPs-CD-DNA
[0048] S2. Take 20 μL of the carbon dot stock solution prepared in step (1), dilute to 500 μL, and centrifuge at 12500 rpm for 20 min to prepare a carbon dot base solution for use.
[0049] S3. Add 20 μL 10 to the above carbon dot base solution -5 M 5' end-modified carboxyl group initiating DNA chain and 2 mg EDC, mixed with a mixer, and activated at room temperature for 15 min to obtain ...
Embodiment 2
[0067] Example 2: Detection of different concentrations of CEA by DNA carbon dots-silicon nanohydrogels
[0068] 1. Take 100 μL of the SiNPs-CD-MA-DNA carrier solution prepared in Example 1 into 18 centrifuge tubes, respectively numbered a to r.
[0069] 2. Add 20μL of 0ng / mL, 4x10 -6 ng / mL, 7x10 - 6 ng / mL, 1x10 -5 ng / mL, 4x10 -5 ng / mL, 7x10 -5 ng / mL, 1x10 -4 ng / mL, 4x10 -4 ng / mL, 7x10 -4 ng / mL, 1x10 -3 ng / mL, 4x10 -3 ng / mL, 7x10 -3 ng / mL, 1x10 -2 ng / mL, 4x10 -2 ng / mL, 7x10 -2 ng / mL, 0.1ng / mL, 0.4ng / mL, 1.0ng / mL analyte CEA standard solution.
[0070] 3. Put the above centrifuge tubes into which different concentrations of CEA standard solutions were added in sequence, and place them in a shaker at 37°C for 3 hours of shaking reaction.
[0071] 4. After the time is up, take out the above centrifuge tubes from the shaker, take 50 μL of the reaction solution from each centrifuge tube and place it in a fluorescence spectrophotometer cuvette. interval of the emissi...
Embodiment 3
[0074] Example 3: Fluorescence detection of carcinoembryonic antigen based on DNA carbon dots-silicon nanohydrogel materials Fluorescence signal contrast
[0075] Take 100 μL of the DNA carbon dots-silicon nanohydrogel prepared in Example 1 into 6 test tubes, one of which is a blank control, and add 20 μL of 4 test tubes to the concentration of 10 -6 ng / mL of bovine serum albumin (BSA), thrombin (thrombin), alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA), add 20 μL bovine serum albumin (BSA), thrombin to the last tube (thrombin), alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) concentrations were all 10 -6 ng / mL mixed solution, and put it into a shaker to react for 3 hours, and then measured the fluorescence intensity of 6 solutions with a molecular fluorometer. Repeat the experiment for many times and take the average value of the fluorescence intensity for subsequent data analysis.
[0076] The result is as Figure 5 As shown in the figure, the fluor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com