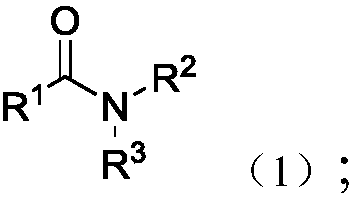
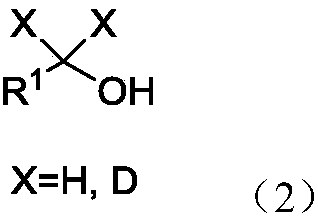
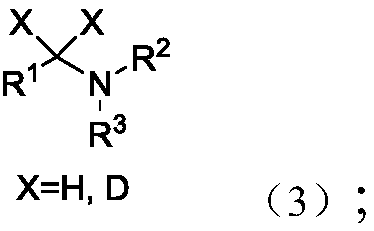Method used for reduction of tertiary amide into alcohols and/or amines
A technology for tertiary amides and tertiary amine compounds is applied in the reduction field of selectively reducing tertiary amides to alcohols and/or tertiary amines, which can solve the problems of difficult to control products, toxic and harmful, harsh conditions, etc., and achieves environmental protection and safe operation. Simple, wide-ranging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064]
[0065] In a 10mL single-necked bottle, under nitrogen protection, add 0.50mmol compound 1a, 2.5mL cyclohexane, 10mmol ethanol, 5.0mmol sodium dispersion reagent (34.1wt%, mineral oil suspension, particle size 5-10μm), 0°C It was stirred at 100°C for 5 min, warmed to room temperature, and quenched with saturated aqueous sodium bicarbonate solution. Diethyl ether and saturated brine were added for extraction, the organic phase was dried, concentrated, and separated by column chromatography to obtain 32 mg of the target compound 2a with a yield of 47%.
[0066] 1 H NMR (400MHz, CDCl 3 )δ7.34-7.29(m,2H),7.25-7.19(m,3H), 3.69(t,J=6.3Hz,2H),2.73(t,J=7.8Hz,2H),1.96-1.88(m ,2H),1.76 (br,1H); 13 C NMR (100MHz, CDCl 3 )δ141.9, 128.5, 128.4, 125.9, 62.3, 34.2, 32.1.
Embodiment 2
[0068]
[0069] In a 10mL single-necked bottle, add 0.50mmol compound 1a, 2.5mL toluene, saturated aqueous sodium bicarbonate (H 2 (0:10mmol), 2.0mmol sodium dispersion reagent (34.1wt%, toluene suspension, particle size<100μm), stirred at 0°C for 30min, raised to room temperature, and quenched the reaction with saturated aqueous sodium bicarbonate solution. Diethyl ether and saturated brine were added for extraction, the organic phase was dried, concentrated, and separated by column chromatography to obtain 14 mg of the target compound 3a with a yield of 15%.
[0070] 1 H NMR (300MHz, CDCl 3 )δ7.31-7.22(m,2H),7.21-7.13(m,3H), 2.65(t,J=7.8Hz,2H),2.53-2.41(m,6H),1.84(m,2H),1.80 -1.71(m, 4H); 13 C NMR (75MHz, CDCl 3 )δ142.3, 128.4, 128.3, 125.7, 56.1, 54.2, 34.0, 30.7, 23.5.
Embodiment 3
[0072]
[0073] In a 10mL single-necked bottle, add 0.50mmol compound 1a, 2.5mL toluene, saturated aqueous sodium bicarbonate solution (D 2 O: 10mmol, 2.0mmol sodium dispersion reagent (34.1wt%, toluene suspension, particle size <100μm), stirred at 0°C for 30min, raised to room temperature, quenched with saturated aqueous sodium bicarbonate solution. Diethyl ether and saturated brine were added for extraction, the organic phase was dried, concentrated, and separated by column chromatography to obtain 28.7 mg of the target compound 5a with a yield of 30%.
[0074] 1 H NMR (300MHz, CDCl 3 )δ7.32-7.22(m,2H),7.22-7.13(m,3H), 2.65(t,J=7.6Hz,2H),2.48(m,4H),1.84(t,J=7.6Hz,2H ),1.77(m, 4H); 13 C NMR (75MHz, CDCl 3 )δ142.3, 128.4, 128.3, 125.7, 55.2(m), 54.1, 33.9, 30.4, 23.5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com