Stable isotope labeled ethyl 3-aminobenzoate-d 5 and its preparation method
An aminobenzoic acid and isotope labeling technology, which is applied in the direction of isotope introduction into organic compounds, acyclic/carbocyclic compound isotope introduction, organic chemical methods, etc., can solve the problems of high detection limit of high performance liquid chromatography and spectrophotometry, and achieve The process route is simple, the promotion prospect is good, and the effect of easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
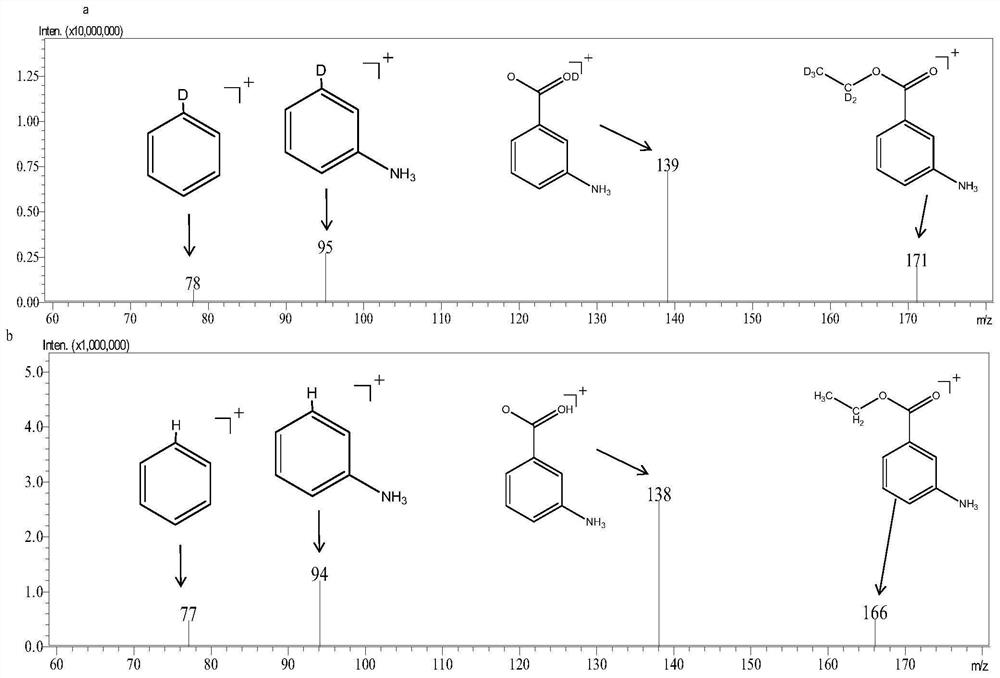
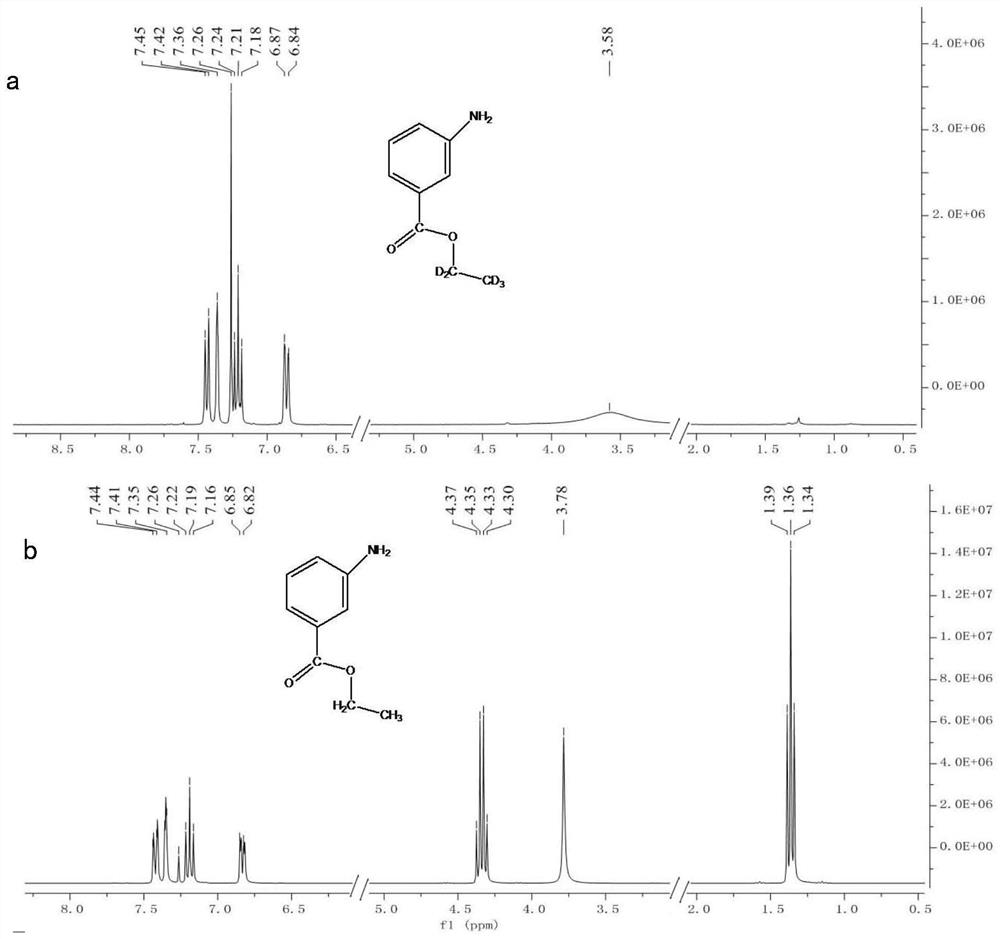
[0031] Measure 5mL (85.6mmol) ethanol-D 6 (DOCD 2 cd 3 ) and 0.5g (3.3mmol) 3-aminobenzoic acid were successively added into the round-bottomed flask; the round-bottomed flask was placed in an ice bath, and under magnetic stirring conditions, 0.5mL thionyl chloride was added dropwise; after completion, the Place the round-bottomed flask at room temperature and continue magnetic stirring for 2 hours; after the reaction is complete, concentrate by rotary evaporation to a volume of about 1 mL; cool to room temperature, add 150 mL of water; add 10 mL of ethyl acetate for extraction, repeat the extraction three times, and combine ethyl acetate for extraction solution; add 50mL water to wash the ethyl acetate extract, and repeat the washing three times; after concentrated by rotary evaporation, use silica gel chromatography column for separation and purification to obtain stable isotope labeled ethyl 3-aminobenzoate-D 5 .
[0032] Ethyl 3-aminobenzoate-D by HPLC-MS using ion sour...
Embodiment 2
[0034] 1 Material method
[0035] 1.1 Instrument
[0036] 20At high performance liquid chromatography (Shimadzu), 8060 triple quadrupole mass spectrometer (Shimadzu), etc.
[0037] 1.2 Reagents
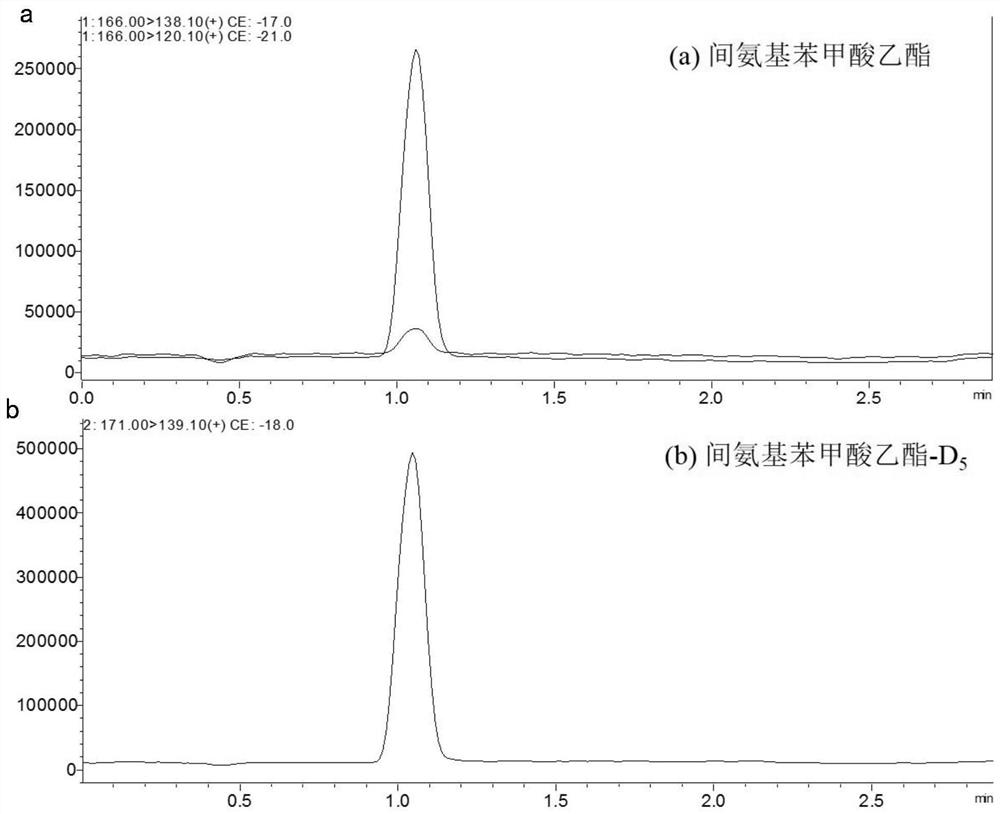
[0038] Acetonitrile (chromatographically pure, purchased from Merck, Germany), sodium chloride and anhydrous magnesium sulfate (analytical pure, purchased from Beijing Chemical Plant), PSA (40-63 μm, Purchased from Shanghai Anpu Experiment Technology Co., Ltd.), ethyl 3-aminobenzoate (imported, purity>98%), ethyl 3-aminobenzoate-D 5 (laboratory synthesis), ethyl 3-aminobenzoate and ethyl 3-aminobenzoate-D 5 Standard solutions were prepared in methanol.
[0039] 1.3 Chromatographic conditions
[0040] a) Chromatographic column: Kromasil C18 reverse-phase chromatographic column ((2.1mm×50mm, 3.5μm);
[0041] b) Mobile phase and gradient elution conditions:
[0042]At the end of 0-1.5min, the volume percentage of mobile phase 0.1% formic acid aqueous solution is from 60% to 10%, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com