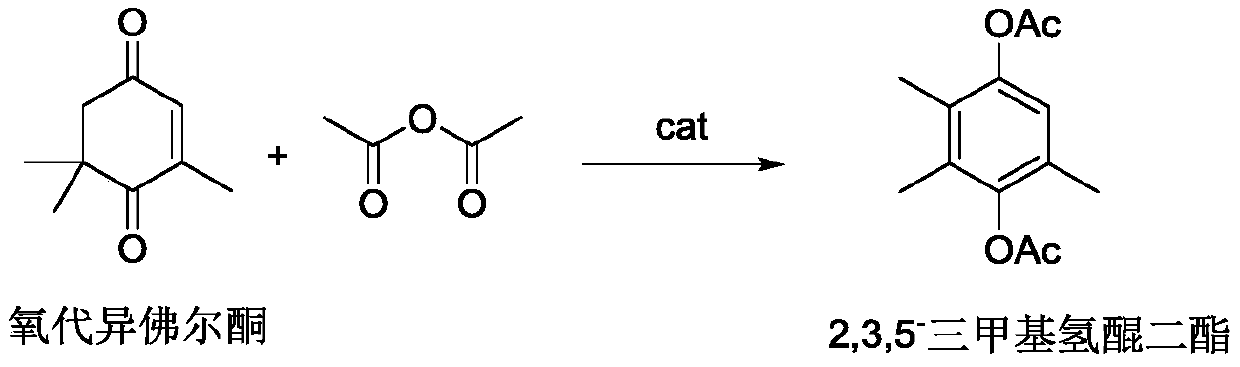
Preparation method of 2,3,5-trimethyl hydroquinone diester
A technology of trimethylhydroquinone diester and carboxymethyl, which is applied in the field of preparation of 2,3,5-trimethylhydroquinone diester by rearrangement of oxoisophorone, which can solve the reaction conversion rate Only 38%, cumbersome catalyst preparation process, unsatisfactory product selectivity and other problems, to achieve the effect of catalytic efficiency and specificity, green environmental protection and three wastes, and inhibit the migration of hydroxyl groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 10g of carboxymethyl-β-cyclodextrin and 80mL of polyallylamine solution (molecular weight 5000, concentration 20wt%, viscosity 13mpa·s) were fully stirred and reacted for 8h in a constant temperature shaker at 60℃. After the reaction was completed Washed with deionized water for 3 times, and then dried under vacuum to obtain polyallylamine modified carboxymethyl-β-cyclodextrin (PAA-CM-β-CD), IR: 1656.01cm- 1 (Secondary amide C=O), 1556.99cm- 1 (Secondary amide NH bond).
[0044] Weigh 5g PAA-CM-β-CD, 10g SO 4 2- / ZrO 2 The solid acid and 100g ethanol were placed in a 250ml three-necked flask, mechanically stirred at 20°C for 5h, filtered with suction, and the filter cake was vacuum dried to obtain a modified cyclodextrin-supported solid acid catalyst (denoted as catalyst a), IR: 1656.01cm- 1 (Secondary amide C=O), 1556.99cm- 1 (Secondary amide NH bond), 1586.08cm- 1 (NH 2 + ).
Embodiment 2
[0046] Mix 20g of carboxymethyl-β-cyclodextrin and 160mL of polyallylamine solution (molecular weight 5000, concentration 20wt%, viscosity 13mpa·s) in a constant temperature shaker at 60°C for 8 hours. After the reaction, Washed with deionized water for 3 times, and then dried under vacuum to obtain polyallylamine modified carboxymethyl-β-cyclodextrin (PAA-CM-β-CD), IR: 1656.06cm- 1 (Secondary amide C=O), 1556.96cm- 1 (Secondary amide NH bond).
[0047] Weigh 20g PAA-CM-β-CD, 10g SO 4 2- / ZrO 2 The solid acid and 200g ethanol were placed in a 500ml three-necked flask, mechanically stirred at 40°C for 1h, filtered with suction, and the filter cake was vacuum dried to obtain a modified cyclodextrin-supported solid acid catalyst (denoted as catalyst b), IR: 1656.01cm- 1 (Secondary amide C=O), 1556.99cm- 1 (Secondary amide NH bond), 1586.18cm- 1 (NH 2 + ).
Embodiment 3
[0049] 10g of carboxymethyl-β-cyclodextrin and 80mL of polyallylamine solution (molecular weight 5000, concentration 20wt%, viscosity 13mpa·s) were fully stirred and reacted for 8h in a constant temperature shaker at 60℃. After the reaction was completed Wash 3 times with deionized water, and then vacuum dry to obtain polyallylamine modified carboxymethyl-β-cyclodextrin (PAA-CM-β-CD) IR: 1655.91cm- 1 (Secondary amide C=O), 1556.92cm- 1 (Secondary amide NH bond).
[0050] Weigh 5g PAA-CM-β-CD, 10g SO 4 2- / ZrO 2 Solid acid and 100g ethanol were placed in a 250ml three-necked flask, mechanically stirred at 30°C for 2h, filtered with suction, and the filter cake was vacuum dried to obtain a modified cyclodextrin-supported solid acid catalyst (denoted as catalyst c), IR: 1656.02cm- 1 (Secondary amide C=O), 1556.98cm- 1 (Secondary amide NH bond), 1586.12cm- 1 (NH 2 + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com