Simple and convenient production method of avibactam
A compound and solvent technology, applied in the field of simple and convenient preparation of avibactam, can solve the problems of unfavorable industrial production, high starting material price, low atom utilization rate and the like, and achieves low cost, high atom utilization rate and simple preparation steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
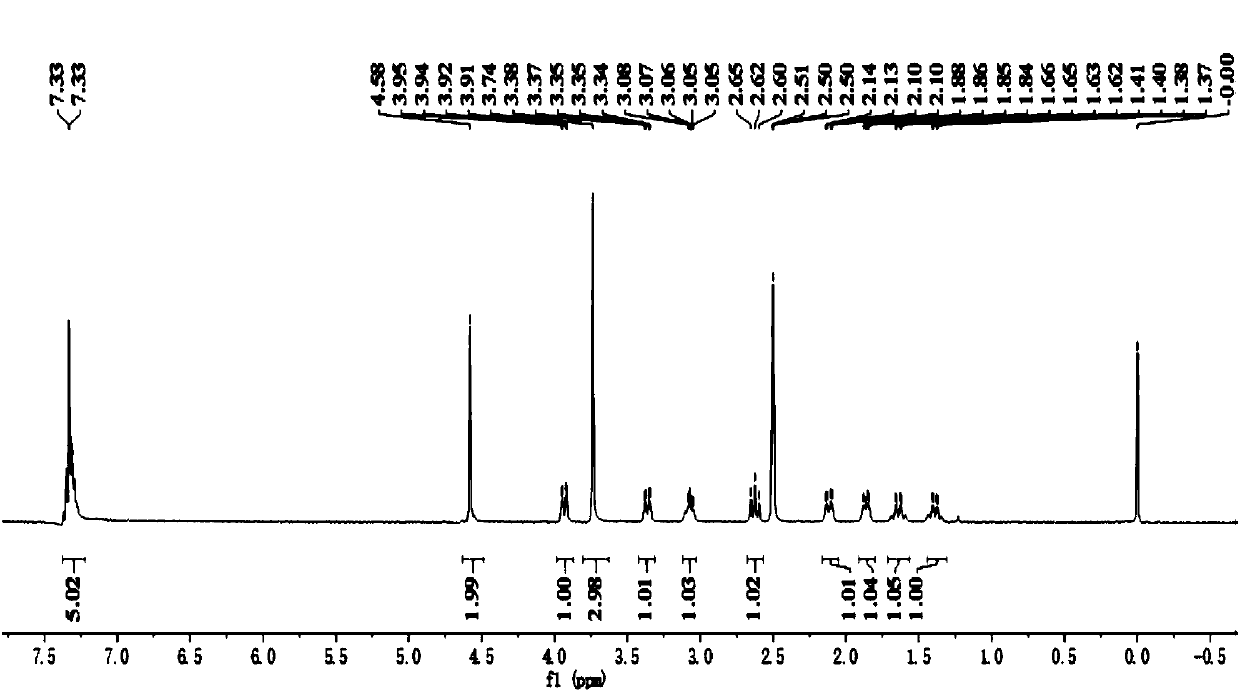
[0089] Example 1: Preparation of Avibactam (Ⅰ)
[0090] Step (1): 5-Methoxymethyloxyiminopiperidine-2S-methyl formate (Ⅲ 1 ) Preparation
[0091] Into a 500ml four-necked flask equipped with a stirrer, a thermometer and a reflux condenser were added 200g of 1,2-dichloroethane, 23.5g (0.15mol) of piperidin-5-one-2S-methyl formate, 20.5 G (0.18 mol) of methoxymethyl hydroxylamine hydrochloride, 25 g of triethylamine, stirred at 40-45°C for 4 hours, cooled to 20-25°C, added 100 g of water, layered, and the water layer used 1, Extract with 2-dichloroethane twice, each with 50 grams of 1,2-dichloroethane, combine the organic layers, and wash twice with saturated brine, each with 25 grams.
[0092] After the solvent was recovered from the organic phase, it was distilled under reduced pressure to obtain 31.3 g of light yellow liquid 5-methoxymethyloxyiminopiperidine-2S-methyl formate, with a GC purity of 99.8% and a yield of 96.5%.
[0093] Step (2): 5R-methoxymethyloxyaminopiperidine-2S-me...
Embodiment 2
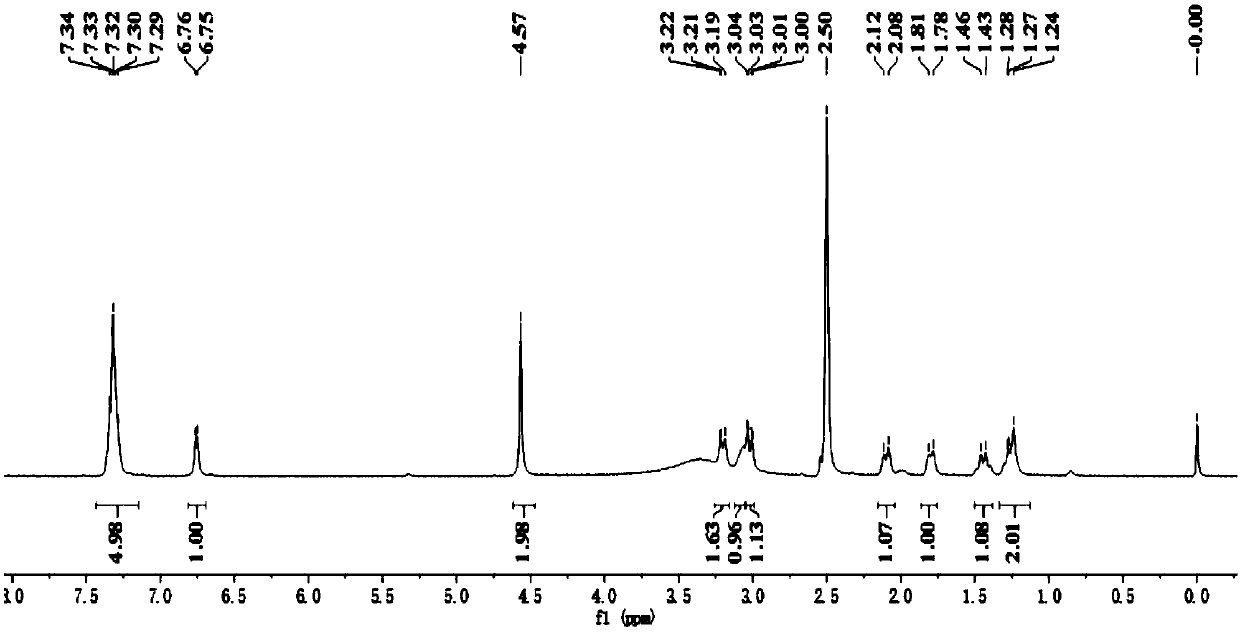
[0115] Example 2: Preparation of Avibactam (Ⅰ)
[0116] Step (1): 5-tert-butyldimethylsilyloxyiminopiperidine-2S-methyl formate (Ⅲ 2 ) Preparation
[0117] Into a 500 ml four-necked flask equipped with a stirrer, a thermometer and a reflux condenser were added 200 g of dichloromethane, 23.5 g (0.15 mole) of piperidin-5-one-2S-methyl formate, 36.5 g (0.2 mole) Tert-butyl dimethylsilyl hydroxylamine hydrochloride, 25 g of triethylamine, stirred at 38-40°C for 5 hours, cooled to 20-25°C, added 100 g of water, layered, and the aqueous layer was extracted with dichloromethane Twice, 50 grams of dichloromethane each time, combine the organic layers, and wash twice with saturated brine, 25 grams each time. After the solvent was recovered from the organic phase, it was distilled under reduced pressure to obtain 41.0 g of light yellow liquid 5-tert-butyldimethylsilyloxyiminopiperidine-2S-methyl formate, with a GC purity of 99.9% and a yield of 95.6%.
[0118] Step (2): 5R-tert-butyldimethyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com