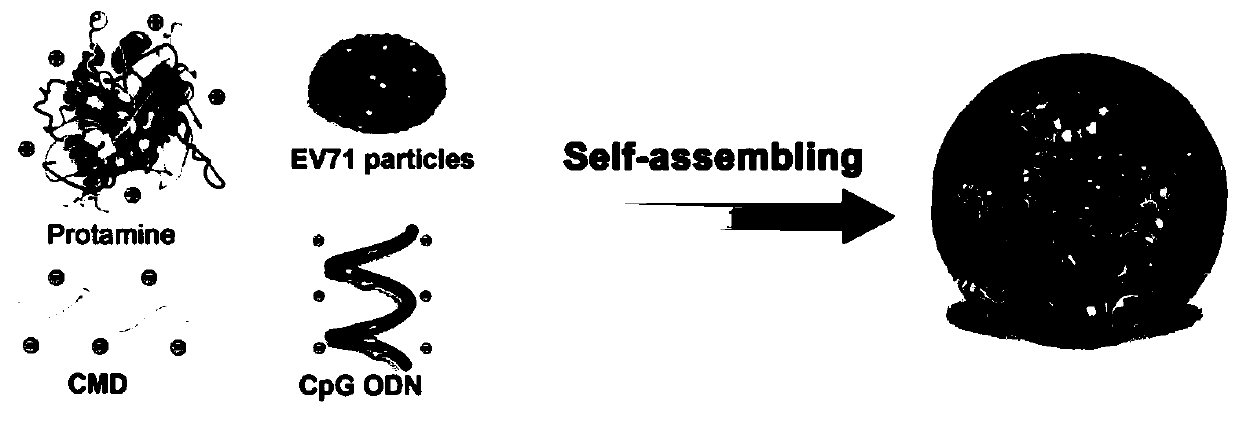
Self-assembly nanometer adjuvant and preparing method and application of nanometer vaccine formed by self-assembly nanometer adjuvant
A nano-vaccine and self-assembly technology, which is applied in the preparation of nano-vaccine and in the field of nano-vaccine, can solve problems such as application limitations, achieve good delivery effect, enhance bioavailability, and maintain strong immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
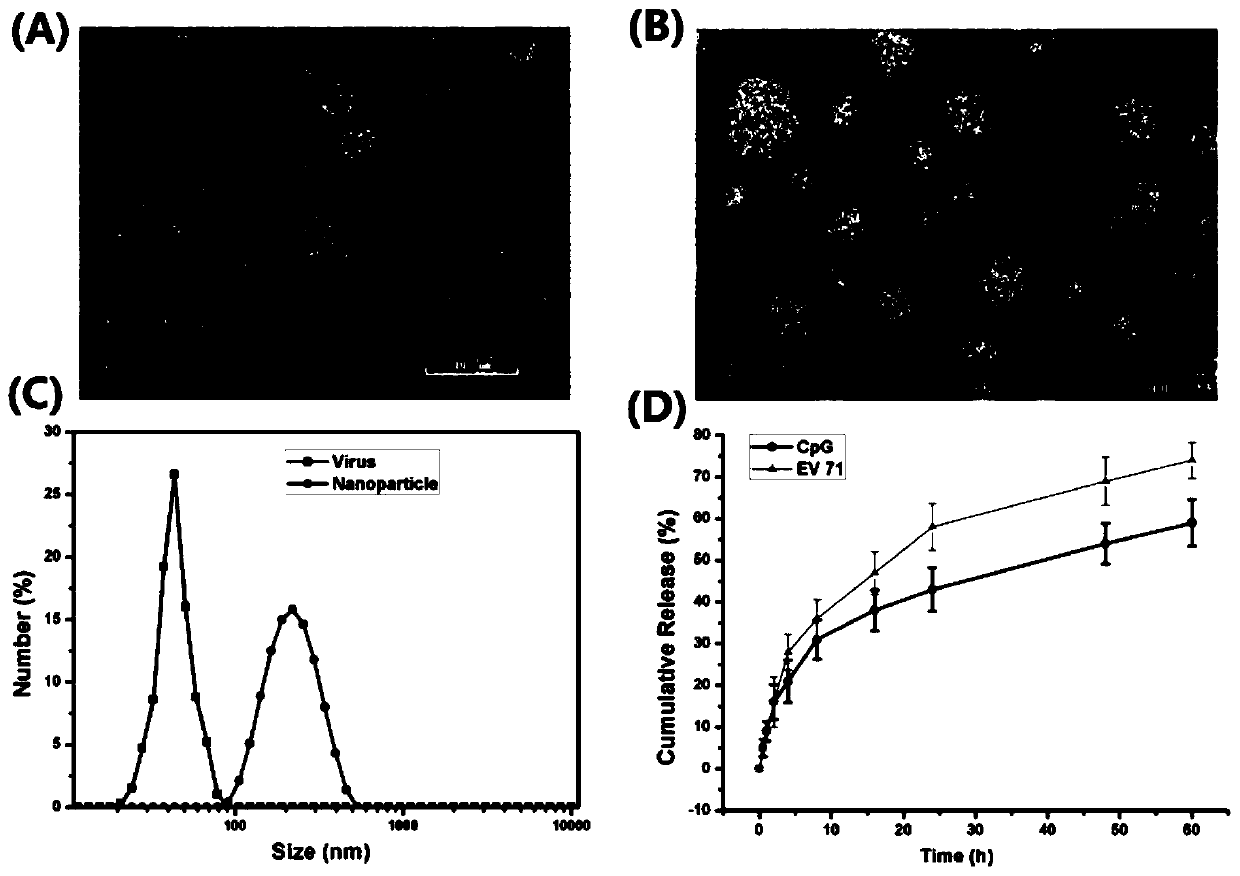
[0042] This example is the preparation process of EV71 nano vaccine formed by encapsulating EV71 inactivated virus with nano adjuvant disclosed in the present invention.
[0043](a) the preparation method comprises the following steps:
[0044] (1) Prepare 2 mg / mL carboxymethyl dextran solution with deionized water, and pass through a 0.22 μm microporous membrane;
[0045] (2) Prepare a 1 mg / mL protamine sulfate solution with deionized water, and pass through a 0.22 μm microporous membrane;
[0046] (3) dissolving the EV71 inactivated virus and CpG oligodeoxynucleotides in the carboxymethyl dextran solution in the above (1);
[0047] (4) Add the protamine sulfate solution in the above (2) dropwise to the solution in the above (3), finally protamine sulfate: carboxymethyl dextran: EV71 inactivated virus: CpG The mass ratio of oligodeoxynucleotides is 1.2:2:0.075:0.0375. Use a magnetic stirrer to continuously stir for 30 minutes, place it stably, centrifuge, remove the superna...
Embodiment 2
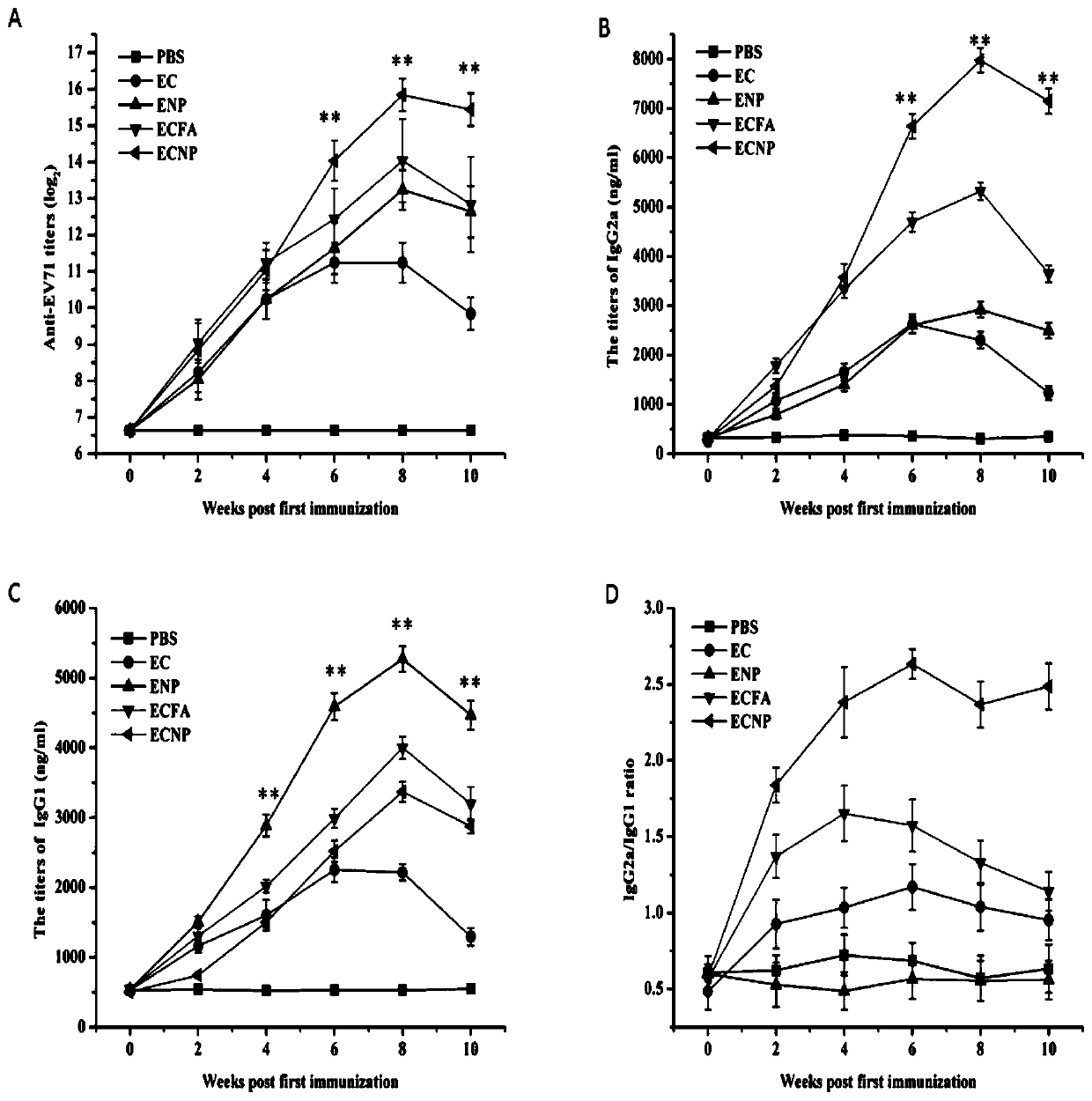
[0053] (a) EV71 inactivated virus group (abbreviated as ENP), EV71 inactivated virus and CpG oligodeoxynucleotide mixed group (abbreviated as EC) with EV71 nano-vaccine (abbreviated as ECNP) prepared in Example 1 and nano self-assembly carrier , EV71 inactivated virus and CpG oligodeoxynucleotide mixed group with incomplete Freund's adjuvant (abbreviated as ECFA) and PBS solution group were used as negative control (abbreviated as PBS) to immunize mice respectively, and the immunization scheme was as follows:
[0054] BALB / c female mice aged 6-8 weeks were selected as immunization objects, and the experimental animals were divided into 5 groups, 8 each in the ECNP group, ENP group, EC adjuvant group, EC group, and PBS group.
[0055] (1) For the first immunization (week 0), the injection volume of each injection is 100 μL. Injected subcutaneously.
[0056] (2) Booster immunization (2 weeks, 4 weeks), the injection volume of each needle is 100 μL, and the concentration is half...
Embodiment 3
[0076] (a) get in embodiment 2, immunize the mouse serum after 6 weeks, measure the content of IFN-α in the serum by ELISA method, concrete steps are as follows:
[0077] (1) Adding samples: Set standard wells, sample wells to be tested and blank wells respectively. Set standard wells to 7 wells, and add 100 μL of standard products with different concentrations in turn. Add 100 μL to the blank well, add 100 μL of the sample to be tested to the remaining well, add a film to the enzyme plate, and incubate at room temperature at 37 degrees for 2 hours.
[0078] (2) Discard the liquid, spin dry without washing.
[0079] (3) Add 100 μL of detection solution A working solution to each well, cover the enzyme plate with a film, and incubate at room temperature at 37 degrees for 1 hour.
[0080] (4) Discard the liquid in the wells, wash each well with 350 μL of washing solution, soak for 1-2 minutes, absorb (do not touch the plate wall) or shake off the liquid in the microplate, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com