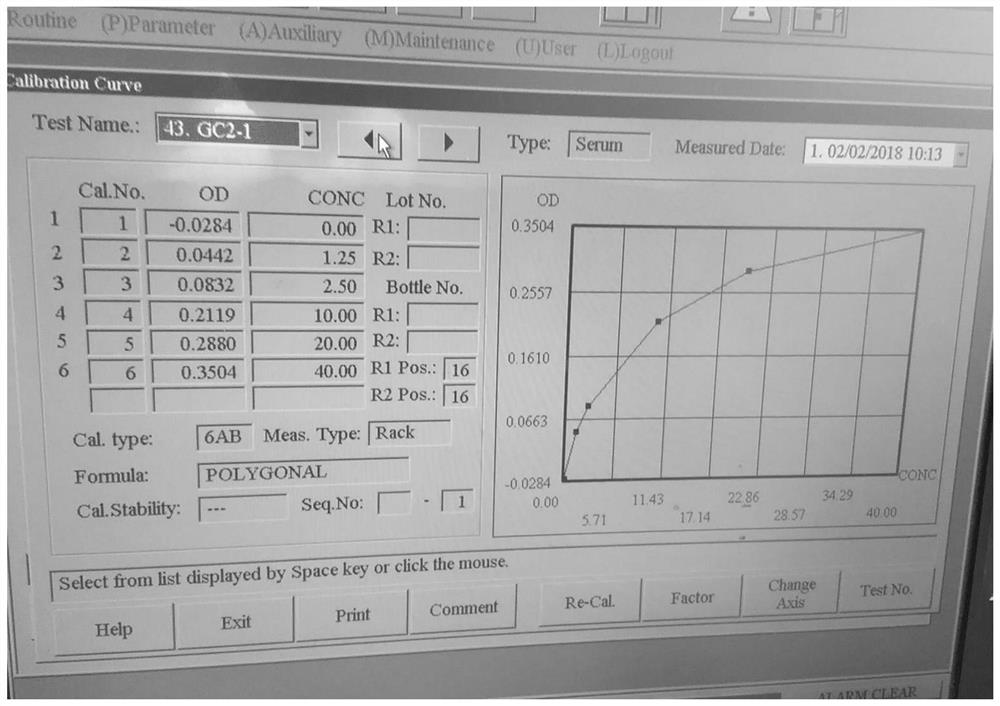
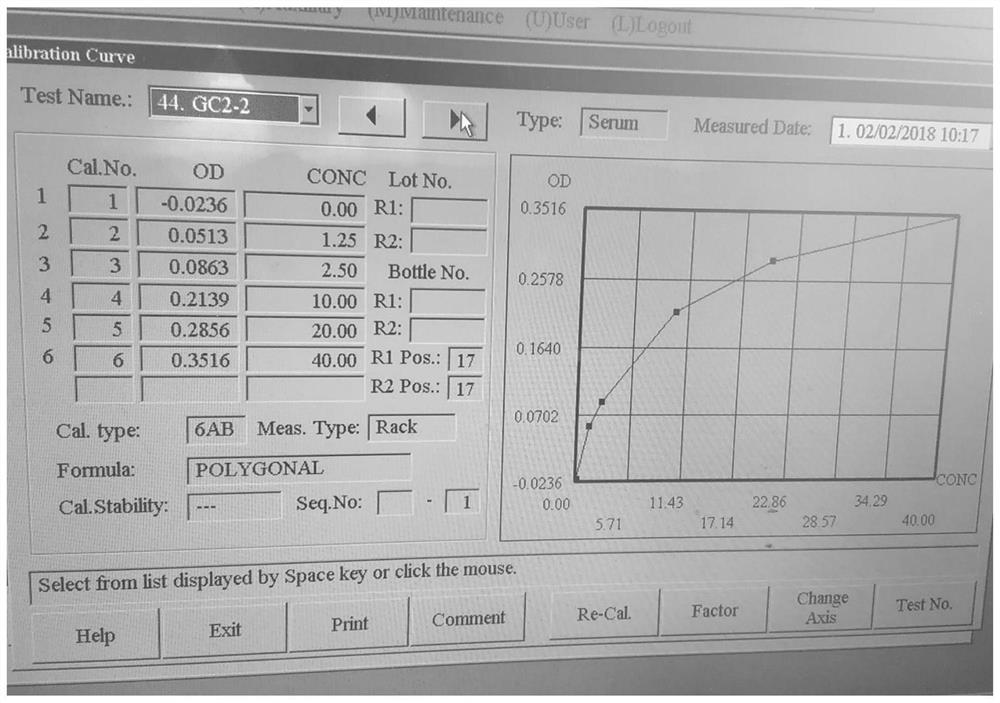
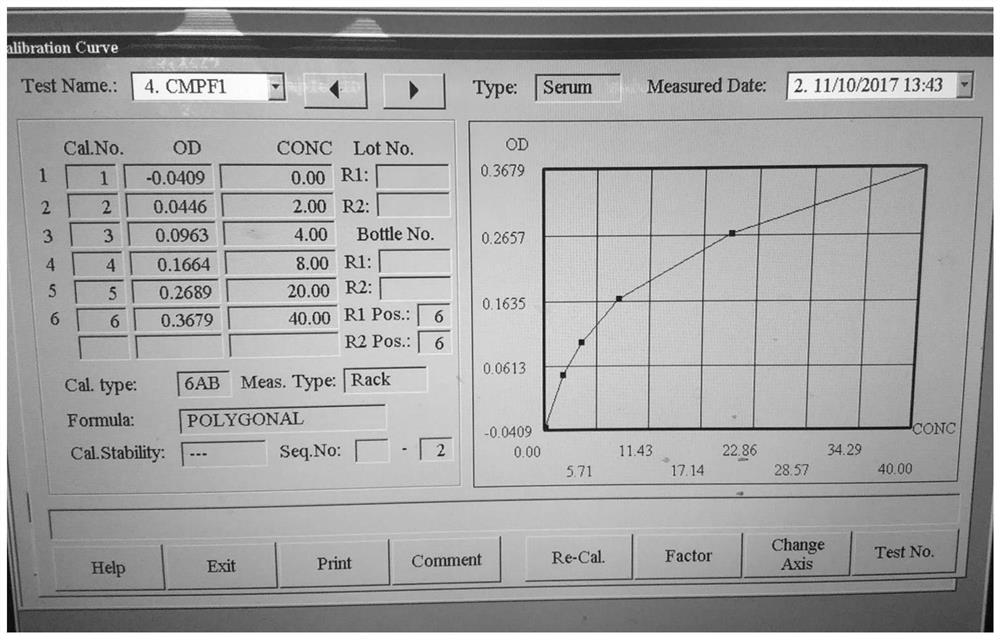
Application of oxidized thio-coenzyme I in homogeneous enzyme immunodiagnostic reagents
A homogeneous enzyme immunoassay and diagnostic reagent technology, applied in biological testing, instruments, measuring devices, etc., can solve the problems of poor anti-hemoglobin interference, poor accuracy and repeatability, poor stability, etc., to reduce detection costs and weaken Influence, the effect of improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of Glycocholic Acid Reagent:
[0047] (1) Preparation of reagent R1 and reagent R2
[0048] (1-1) Accurately weigh 1 L of prepared (30-70) mM tris buffer, fully dissolve and mix well, and adjust the pH to 6.0-7.0;
[0049] (1-2) Take 800 ml of buffer solution in step 1-1, add (4-5) mM G6P, 0.05% pc-300, (1.2-2.0) KU / L glycocholic acid monoclonal antibody successively, fully Dissolve and mix well for later use;
[0050] (1-3) Take 400ml of the solution in step 1-2, add (4-6)mM oxidized thio-coenzyme I, mix evenly, keep it at 4°C for overnight use, and mark it as R1;
[0051] (1-4) Take 200ml of buffer solution in step 1-1, add (0.15-0.2)% BSA, (1.5-2)% NaCl, 0.05% PC-300, (0.5-0.6) KU / L glucose hexaphosphate in sequence The dehydrogenase-glycocholic acid conjugate is labeled R2.
[0052] (2) Preparation of calibrators and quality control products
[0053] (2-1) Weigh (0.7-0.9)% NaCl, (0.05-0.06)% pc-300, dissolve in purified water, and prepare as a speci...
Embodiment 2
[0092] Preparation of CMPF reagent:
[0093] (1) Preparation of reagent R1 and reagent R2
[0094] (1-1) Accurately weigh 1 L of prepared (30-70) mM tris buffer, fully dissolve and mix well, and adjust the pH to 6.0-7.0;
[0095] (1-2) Take 800ml of the buffer in step 1-1, add (4-5)mM G6P, 0.05% pc-300, (1.2-2.0)KU / L CMPF antibody in sequence, fully dissolve and mix well for backup use;
[0096] (1-3) Take 400ml of the solution in step 1-2, add (4-6)mM oxidized thio-coenzyme I, mix evenly, keep it at 4°C for overnight use, and mark it as R1;
[0097] (1-4) Take 200ml of buffer solution in step 1-1, add (0.15-0.2)% BSA, (1.5-2)% NaCl, 0.05% PC-300, (0.5-0.6) KU / L glucose hexaphosphate in sequence The dehydrogenase-CMPF conjugate is labeled R2.
[0098](2) Preparation of calibrators and quality control products
[0099] (4-1) Weigh (0.7-0.9)% NaCl, (0.05-0.06)% pc-300, dissolve with purified water, and prepare it as a special diluent for CMPF calibration quality control pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com