Method for separating and extracting iron and nickel from iron and nickel leaching agent
A technology of leaching solution and treatment method, which is applied to the improvement of process efficiency, photographic process, instruments, etc., can solve the problems of expensive investment, poor product purity, high acid consumption, etc., and achieve low process operation cost, excellent economic benefits, and resources The effect of high utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
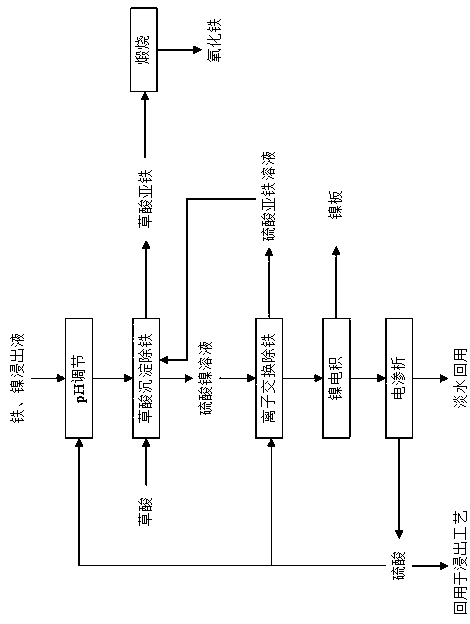
[0027] (1) The iron and nickel leaching solution obtained from the smelting and leaching of nickel sulfide ore is mainly composed of iron sulfate and nickel sulfate, the concentration of sulfuric acid is 0.05%, pH=0.5, Fe:Ni (mass concentration)=2:1, and the concentration of Ni is 5g / L;
[0028] (2) The pH of the iron and nickel leaching solution is first adjusted, and the pH is adjusted to 0.5-1;
[0029] (3) Add oxalic acid to the feed liquid after pH adjustment to remove iron, and the obtained ferrous oxalate is precipitated and calcined to produce iron oxide, and the purity of iron oxide is >99.5%;
[0030] Oxalic acid: Fe (molar ratio) = 1:1, the reaction time is 25min, and the reaction temperature is room temperature;
[0031] (4) The iron ion in the nickel sulfate solution obtained after iron precipitation with oxalic acid is 10mg / L, and the ion exchange method is used to further remove iron deeply, and the iron content in the effluent is less than 0.005mg / L. The ferr...
Embodiment 2
[0036] (1) The iron and nickel leaching solution obtained from the smelting and leaching of nickel sulfide ore is mainly composed of iron sulfate and nickel sulfate, the concentration of sulfuric acid is 1.5%, pH=2, Fe:Ni (mass concentration)=4:1, and the concentration of Ni is 20g / L;
[0037] (2) The pH of the iron and nickel leaching solution is first adjusted to 1;
[0038] (3) Add oxalic acid to the feed liquid after pH adjustment to remove iron, and the obtained ferrous oxalate is precipitated and calcined to produce iron oxide, and the purity of iron oxide is >99.5%;
[0039] Oxalic acid: Fe (molar ratio) = 1.5:1, the reaction time is 40min, and the reaction temperature is room temperature;
[0040] (4) The iron ion in the nickel sulfate solution obtained after iron precipitation with oxalic acid is 40mg / L, and the ion exchange method is used to further remove iron deeply, and the iron content in the effluent is less than 0.005mg / L. The ferrous sulfate solution obtaine...
Embodiment 3
[0045] (1) The iron and nickel leaching solution obtained from the smelting and leaching of nickel sulfide ore is mainly composed of iron sulfate and nickel sulfate, the concentration of sulfuric acid is 1%, pH=0.8, Fe:Ni (mass concentration)=3:1, and the concentration of Ni is 15g / L;
[0046] (2) The pH of the iron and nickel leaching solution is adjusted to 0.9;
[0047] (3) Add oxalic acid to the pH-adjusted feed solution to remove iron, and the obtained ferrous oxalate is precipitated and calcined to produce iron oxide. The purity of iron oxide is >99.5%; oxalic acid: Fe (molar ratio) = 1.3:1, and the reaction time is 30 minutes. The reaction temperature is normal temperature;
[0048] (4) The iron ion in the nickel sulfate solution obtained after iron precipitation with oxalic acid is 30 mg / L, and the ion exchange method is used to further remove iron deeply, and the iron content in the effluent is less than 0.005 mg / L. The ferrous sulfate solution obtained by ion excha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com