A kind of production method of laurolactam
A technology of laurolactam and cyclododecanone is applied in the production field of high-purity laurolactam, and achieves the effects of being difficult to agglomerate, being convenient for packaging, storage and transportation, and being easy to realize.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
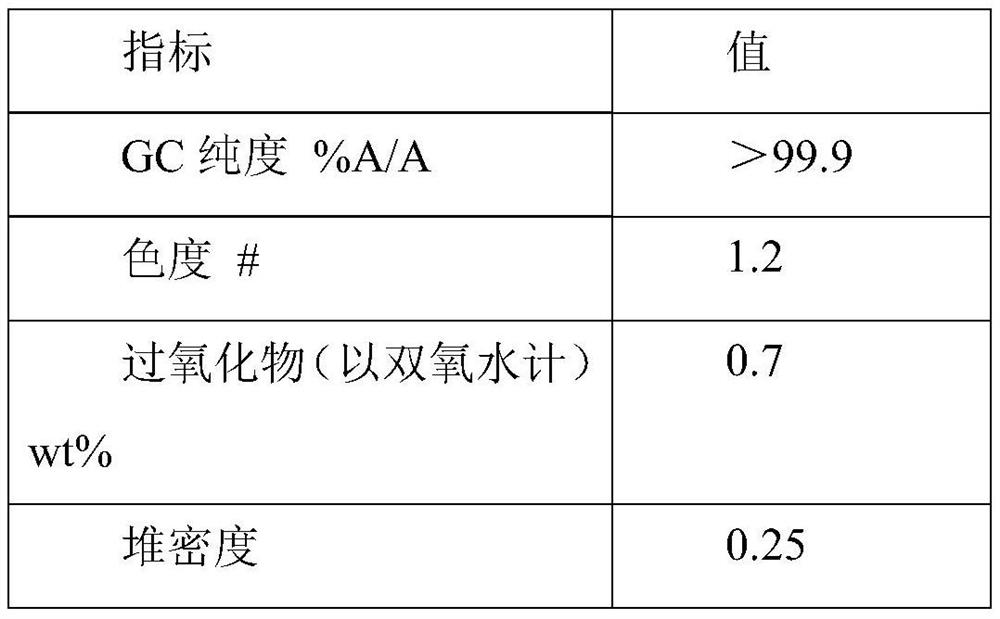
[0043] Step 1: 180 g of an aqueous solution of hydroxylamine sulfate (12.5 wt %) was added to ammonia water to neutralize to pH 6-7 to obtain a hydroxylamine solution. In the round bottom bottle, 100 g of cyclododecanone was heated and melted, kept at 90° C., and the aforementioned hydroxylamine solution was added dropwise into the round bottom bottle while stirring. The dropwise addition time is about 10 minutes. After the dropwise addition is completed, the reaction is continued for 1 hour, and then 100 g of 1-cyclohexyl-n-tridecane is added for liquid separation. The oil phase obtained after liquid separation contained 0.2% water and 100 ppm inorganic salt.
[0044] Step 2: The above-mentioned oil phase is subjected to vacuum rectification, and cyclododecanone is distilled under the conditions of pressure 1kPa, temperature 110°C, and the number of theoretical plates in the rectification column is not less than 20. About 45 g of cyclododecanone was obtained, with a purity o...
Embodiment 2
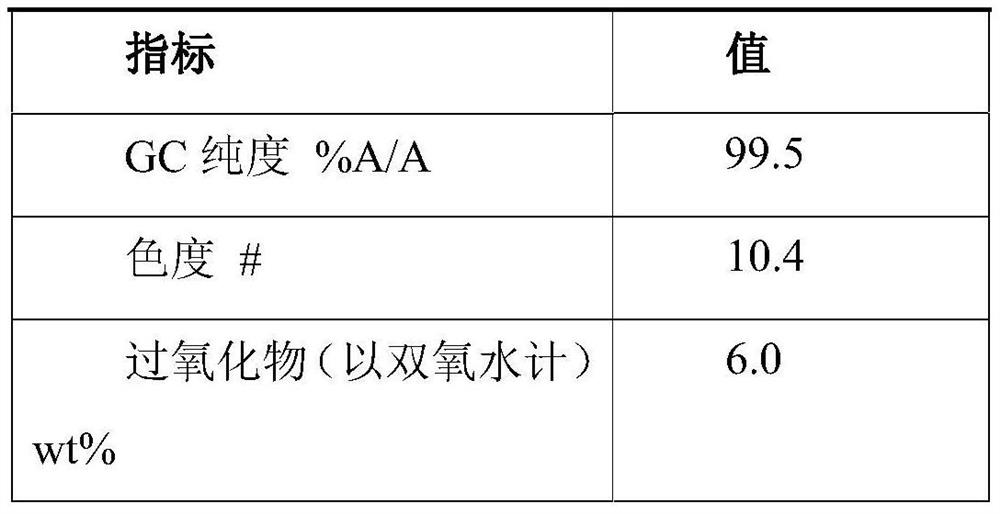
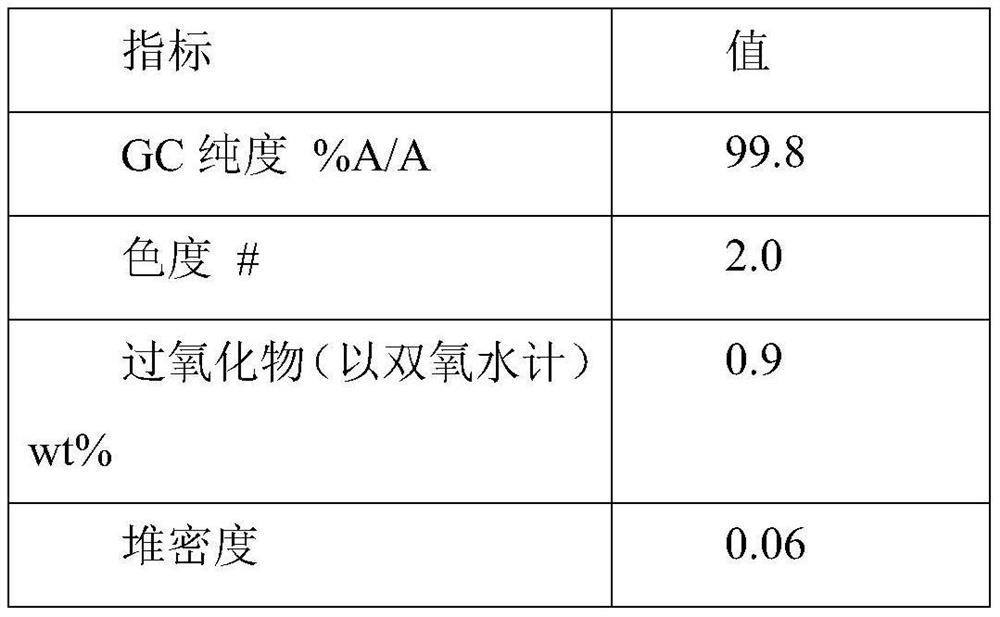
[0067] Step 1: 100 g of an aqueous solution of hydroxylamine sulfate (12.5 wt %) was added to ammonia water to neutralize to pH 6-7 to obtain a hydroxylamine solution. In the round bottom bottle, 100 g of cyclododecanone was heated and melted, kept at 90° C., and the aforementioned hydroxylamine solution was added dropwise into the round bottom bottle while stirring. The dropwise addition time is about 10 minutes. After the dropwise addition is completed, the reaction is continued for 1 hour, and then 120 g of 4-isopropyl-n-decylcyclohexane is added for liquid separation. The oil phase obtained after liquid separation contained 0.15% of water and 80 ppm of inorganic salts.
[0068] Step 2: The above-mentioned oil phase is subjected to vacuum rectification, and cyclododecanone is distilled under the conditions of pressure 1kPa, temperature 110°C, and the number of theoretical plates in the rectification column is not less than 20. About 65 g of cyclododecanone was obtained, wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com