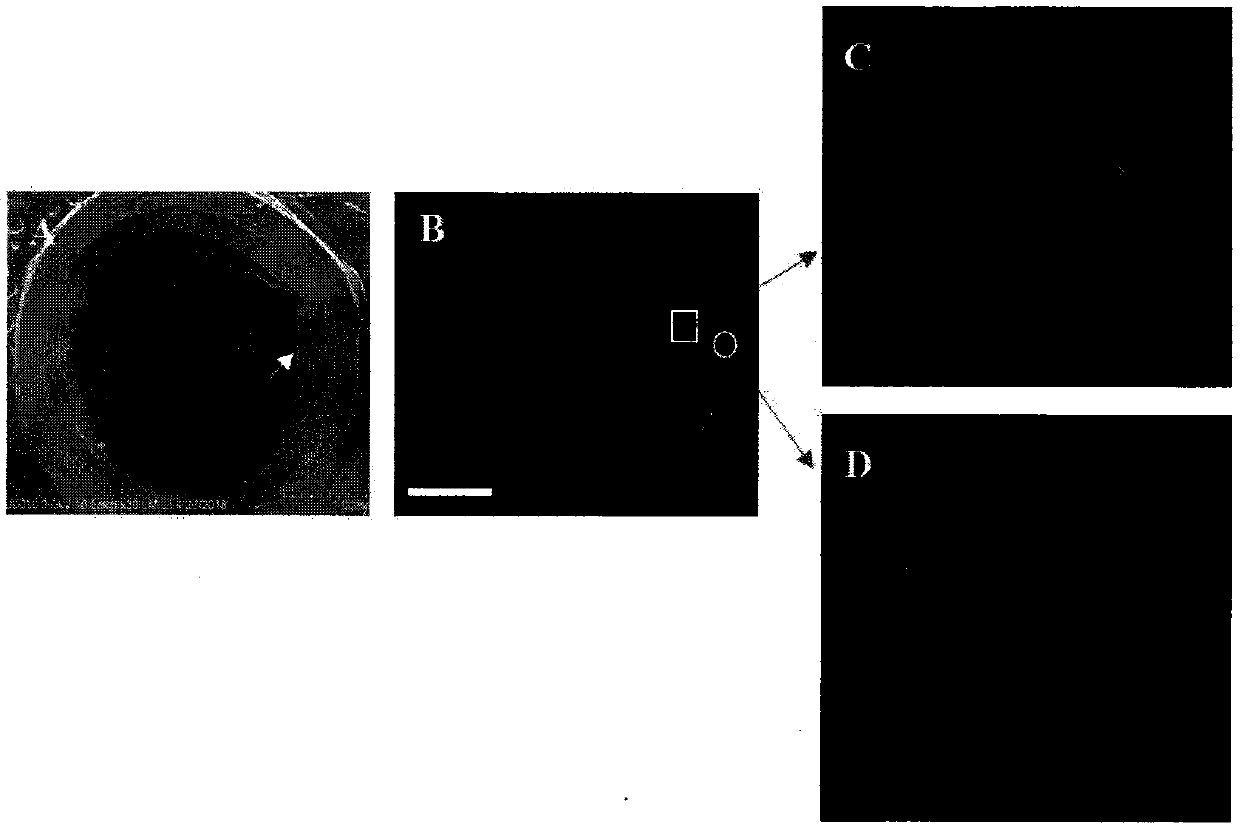
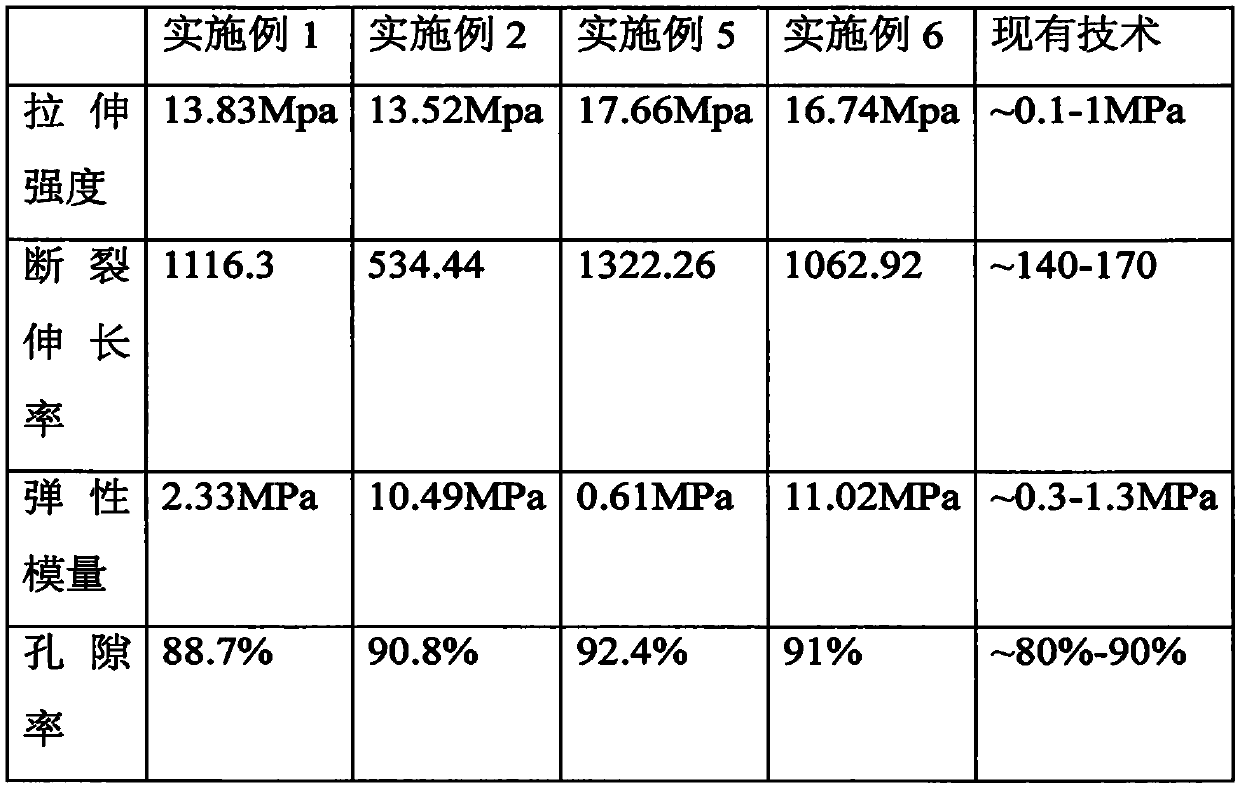
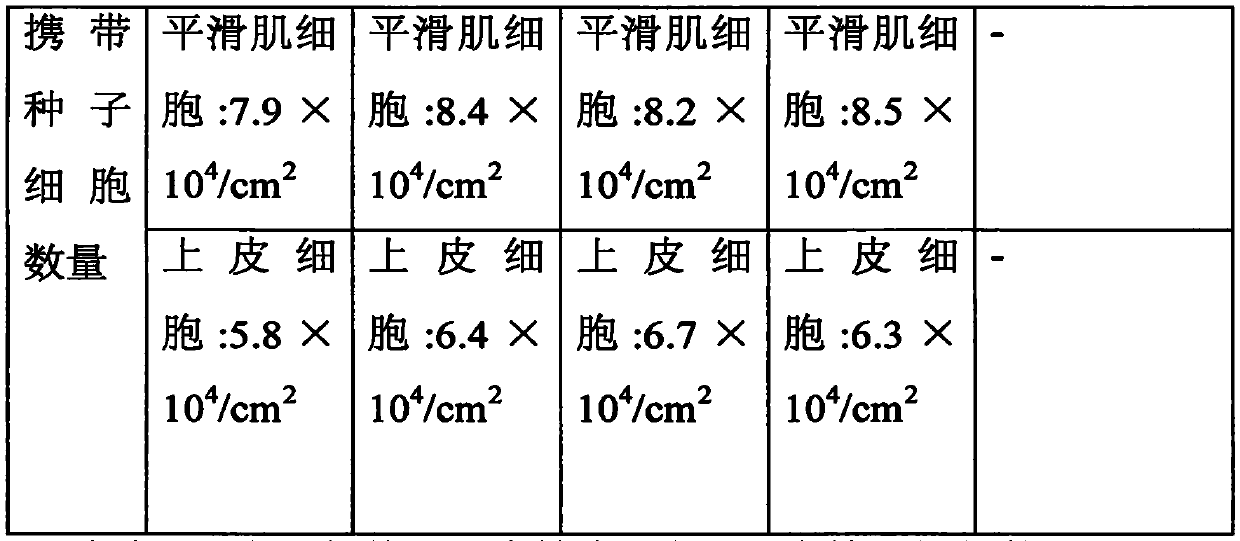
Tissue engineering urethra stent and preparation technology thereof
A tissue engineering and preparation process technology, applied in the field of tissue engineering urethral stent and its preparation technology, can solve the problems of non-damage repair and reconstruction, poor biomechanical properties, difficult quantitative adjustment of pore size and porosity, etc. The effect of good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] This embodiment provides a preparation process for tissue engineering urethral stent, which comprises the following steps:
[0045] Preparation steps of the first electrospinning solution:
[0046] The first solvent is a mixed solvent of chloroform and methanol, and the volume mixing ratio of chloroform and methanol is 3:1; 0.7g PLA and 10mL of the first solvent are mixed in a 50mL single-necked flask to obtain a PLA solution, which is fully dissolved overnight at room temperature. The average molecular weight of PLA is 200,000.
[0047] Preparation steps of the second electrospinning solution:
[0048] The second solvent is a mixed solvent of chloroform and methanol, and the volume mixing ratio of chloroform and methanol is 3:1; mix 1.0 g of gelatin and 10 mL of the second solvent in a 50 mL single-necked flask to obtain a gelatin solution, and fully dissolve at room temperature overnight . Gelatin has an average molecular weight of 150,000.
[0049] Preparation st...
Embodiment 2
[0063] This embodiment provides a preparation process for a tissue-engineered urethral stent, which is roughly the same as the preparation process provided in Example 1, the difference lies in the different parameters, and the difference is as follows:
[0064] Preparation steps of the first electrospinning solution:
[0065] The first solvent is a mixed solvent of hexafluoroisopropanol and trifluoroethanol, and the volume mixing ratio of hexafluoroisopropanol and trifluoroethanol is 3:1; 0.8g PLA and 10mL of the first solvent are mixed in a 50mL single-necked flask to obtain PLA Solution, fully dissolved at room temperature for 4h.
[0066] Preparation steps of the second electrospinning solution:
[0067] The second solvent is a mixed solvent of hexafluoroisopropanol and trifluoroethanol, and the volume mixing ratio of hexafluoroisopropanol and trifluoroethanol is 3:1; mix 11.1g of gelatin with 10mL of the second solvent in a 50mL single-necked flask to obtain gelatin Solu...
Embodiment 3
[0076] This embodiment provides a preparation process for a tissue engineered urethral stent, which is roughly the same as the preparation process provided in Examples 1 and 2, except that the parameters are different, and the differences are as follows:
[0077] Preparation steps of the first electrospinning solution:
[0078] The first solvent is a mixed solvent of tetrahydrofuran and N,N-dimethylformamide, the volume mixing ratio of tetrahydrofuran and N,N-dimethylformamide is 3:1; 0.9g PLA and 10mL of the first solvent are mixed in 50mL Mix in a one-necked flask to obtain a PLA solution, and fully dissolve at room temperature for 4 hours.
[0079] Preparation steps of the second electrospinning solution:
[0080] The second solvent is a mixed solvent of tetrahydrofuran and N,N-dimethylformamide, and the volume mixing ratio of tetrahydrofuran and N,N-dimethylformamide is 3:1; 1.2g of gelatin and 10mL of the second solvent are mixed in 50mL of Mix in a one-necked flask to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com