Synthesis method for Beta-glucuronidase precipitation type fluorometric substrate
A technology of glucuronide and synthesis method, which is applied in the field of β-glucuronidase precipitation type fluorescent substrate synthesis, can solve the problem of low yield of target substance, troublesome deprotection group reaction treatment, β-glucuronidase Difficult to synthesize and other problems, to achieve the effect of mild reaction conditions and easy implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
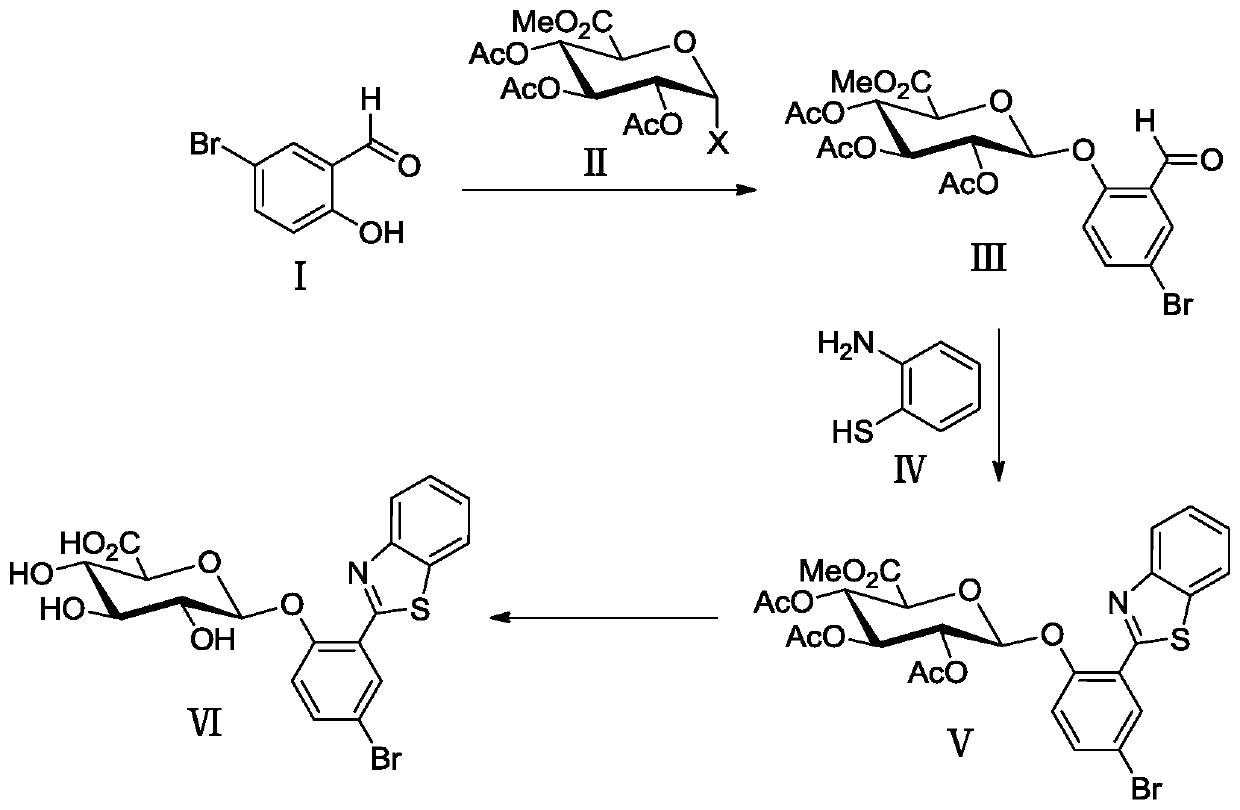
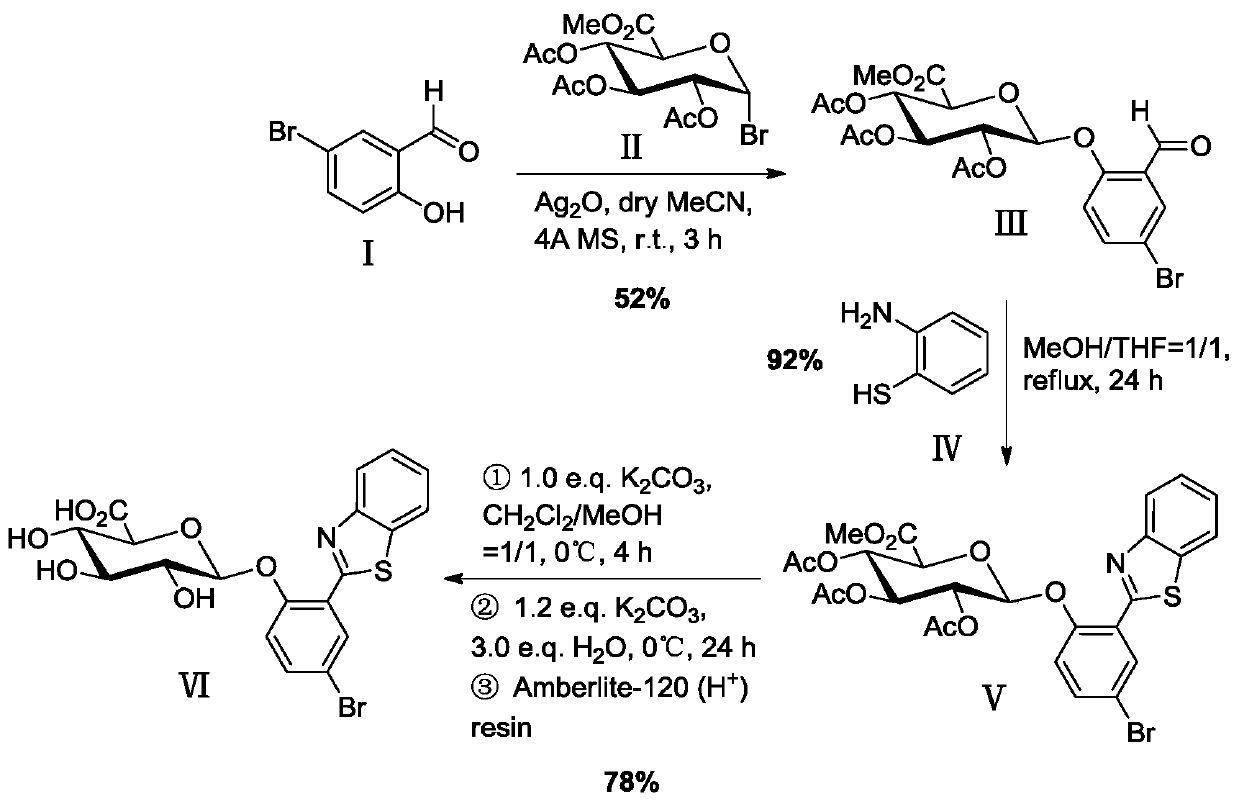
[0037] Example 1 Synthesis of 2-(benzothiazol-2'-yl)-4-bromophenyl-β-D-glucuronide (BTBP-Gluc)
[0038] A kind of synthesis of 2-(benzothiazol-2'-yl)-4-bromophenyl-β-D-glucuronide based on 2-(benzothiazol-2'-yl)-4-bromophenol method, including the following steps
[0039] (1) Glycosylation reaction
[0040] 1.709g (8.50mmol) 5-bromosalicylaldehyde (I), 1.986g (5.00mmol) triacetyl-α-D-methyl bromoglucuronate (II), 1.391g (6.00mmol) silver oxide Placed in 40 mL of dry acetonitrile containing 4.0 g of powdered 4A molecular sieve, stirred and reacted for 3 h in an argon atmosphere, protected from light, and at room temperature. Then, the reaction mixture was suction-filtered with a sand core funnel filled with a dense 300-400 mesh column chromatography silica gel layer, washed with a mixed solution of dichloromethane and ethyl acetate (v / v=2 / 1), and combined the washed The liquid was removed and the solvent was removed by rotary evaporation under reduced pressure, recrystallize...
Embodiment 2
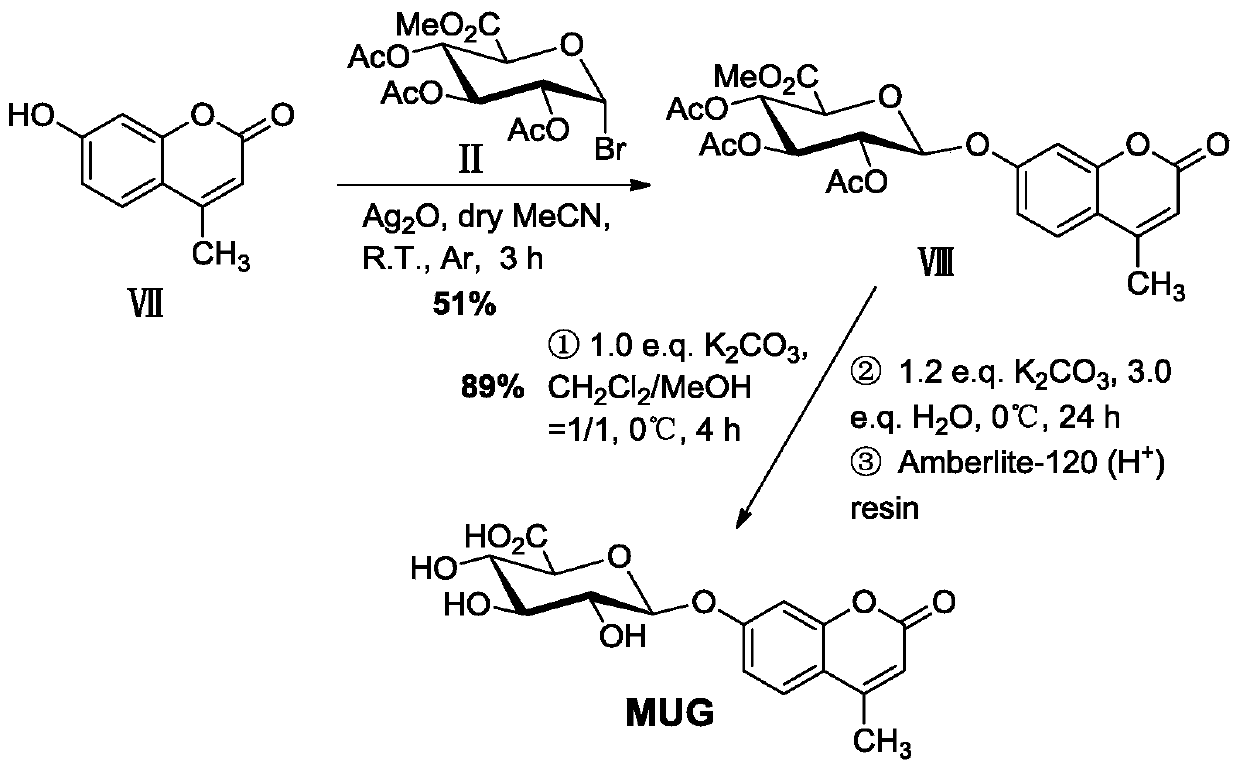
[0054] Example 2 Synthesis of 4-methylumbelliferyl-β-D-glucuronide (MUG)
[0055] A synthetic method of fluorescent substrate 4-methylumbelliferyl-β-D-glucuronide, comprising the following steps:
[0056] (1) Glycosylation reaction
[0057] Oxidize 0.529g (3.00mmol) 4-methylumbelliferone (Ⅶ), 0.794g (2.00mmol) triacetyl-α-D-methyl bromoglucuronate (Ⅱ), 0.556g (2.40mmol) The silver was placed in 16 mL of dry acetonitrile containing 1.6 g of powdered 4A molecular sieve, and stirred for 3 h in an argon atmosphere, protected from light, and at room temperature. Then, the reaction mixture was suction-filtered with a sand core funnel filled with a dense 300-400 mesh column chromatography silica gel layer, washed with a mixed solution of dichloromethane and ethyl acetate (v / v=2 / 1), and combined the washed The liquid was removed and the solvent was removed by rotary evaporation under reduced pressure, recrystallized with methanol, and dried to obtain about 0.506 g (yield about 51%) ...
Embodiment 3
[0067] Example 3 Comparative detection effect of different fluorescent substrates on Escherichia coli ATCC 25922
[0068] The agar plate method was used to detect the localization effect of different fluorescent substrates on Escherichia coli ATCC 25922, and the specific steps were as follows:
[0069](1) Weigh a certain amount of Columbia agar medium and place it in a clean Erlenmeyer flask, add quantitative distilled water, heat to pre-dissolve, and then add quantitative target fluorescent substrate 2-(benzothiazol-2'-yl) - DMSO solution of 4-bromophenyl-β-D-glucuronide (BTBP-Gluc) and common fluorescent substrate 4-methylumbelliferyl-β-D-glucuronide (MUG), making The concentration of the fluorescent substrate is 0.2mmol / L, sealed and sterilized by high-pressure steam at 121°C for 15 minutes, then when the temperature of the medium drops to about 45°C, shake well and quickly pour the plate under sterile conditions, and place it horizontally Allow the medium to solidify for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com