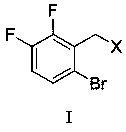
(6-bromo-2, 3-difluorobenzyl) phenyl sulfide and preparation method thereof
A technology of difluorobenzyl and phenyl sulfide, which is applied in the field of (6-bromo-2,3-difluorobenzyl) phenyl sulfide and its preparation, can solve problems such as no literature reports, and achieve selection High performance, high yield, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of (6-bromo-2,3-difluorobenzyl)phenylene sulfide
[0022] Add diphenyl disulfide (1.9 g, 8.75 mmol) into a 100 mL three-neck flask, replace argon three times at room temperature (empty the air), add 10 mL of tetrahydrofuran, add zinc (1.03 g, 15.85 mmol), and Sodium 2g, add water 16 mL. The temperature was raised to 70°C for reflux reaction. After the reflux solution turned from cloudy to clear, 1-bromo-2-(bromomethyl)-3,4-difluorobenzene (5.3 g, 17.6 mmol) was added and the reaction was continued for 4 hours. After the reaction was completed, ethyl acetate was added to the reaction solution for extraction, and the layers were separated into an organic phase and an aqueous phase. After the organic phase was washed with water and dried, the ethyl acetate solvent was concentrated under reduced pressure and spin-dried to obtain 3.46 g of a light yellow liquid. The yield is 60%. Melting point 34.2-36.8°C;
[0023] 1 H NMR (400 MHz, Chloroform-d) δ...
Embodiment 2
[0024] Example 2: Preparation of (6-bromo-2,3-difluorobenzyl)phenylene sulfide
[0025] Add diphenyl disulfide (1.9 g, 8.75 mmol) into a 100 mL three-necked flask, replace argon three times at room temperature (empty the air), add 10 mL of tetrahydrofuran, add sodium borohydride (0.60 g, 15.85 mmol), Sodium hydroxide 2g, add water 16mL. The temperature was raised to 70°C for reflux reaction. After the reflux solution turned from cloudy to clear, 1-bromo-2-(bromomethyl)-3,4-difluorobenzene (5.3 g, 17.6 mmol) was added and the reaction was continued for 4 hours. After the reaction was completed, ethyl acetate was added to the reaction solution for extraction, and the layers were separated into an organic phase and an aqueous phase. After the organic phase was washed with water and dried, the ethyl acetate solvent was concentrated under reduced pressure and spin-dried to obtain 5.59 g of a light yellow liquid. The yield is 95%. Melting point 34.2-36.8°C;
[0026] 1 H NMR (400...
Embodiment 3
[0027] Example 3: Preparation of (6-bromo-2,3-difluorobenzyl)phenylene sulfide
[0028] Add diphenyl disulfide (1.9 g, 8.75 mmol) into a 100 mL three-necked flask, replace argon three times at room temperature (empty the air), add 10 mL of tetrahydrofuran, add sodium borohydride (0.60 g, 15.85 mmol), Ammonia water 10mL. The temperature was raised to 70°C for reflux reaction. After the reflux solution turned from cloudy to clear, 1-bromo-2-(bromomethyl)-3,4-difluorobenzene (5.3g, 17.6 mmol) was added and the reaction was continued for 4 hours. After the reaction was completed, ethyl acetate was added to the reaction solution for extraction, and the layers were separated into an organic phase and an aqueous phase. After the organic phase was washed with water and dried, the ethyl acetate solvent was concentrated under reduced pressure and spin-dried to obtain 2.24 g of a light yellow liquid. The yield is 40%. Melting point 34.2-36.8°C;
[0029] 1 H NMR (400 MHz, Chloroform-d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com