Preparation method of CaF2 hollow nanospheres
A nanomaterial, hollow sphere technology, applied in the direction of calcium/strontium/barium halide, calcium/strontium/barium fluoride, etc., can solve the problems of poor repeatability, uneven crystal size, short synthesis time, etc., to achieve uniform size , the appearance is consistent, the synthesis method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of mixed solution A: using F127 as surfactant, according to the molar ratio of F127 and deionized water of 0.0001, add 1.209g (g is gram, the same below) of F127 into 15ml of deionized water, stir magnetically at room temperature for 1h, and prepare Mixture A is obtained.
[0026] Preparation of mixed solution B: F127:Ca(NO 3 ) 2 :Na 3 C 6 h 5 o 7 :NaBF 4 :H 2 O=0.093:1:0.5:2:838, 0.236g Ca(NO 3 ) 2 4H 2 O, 0.147g Na 3 C 6 h 5 o 7 2H 2 O and 0.219 g NaBF 4 Add to the mixed solution A sequentially every five minutes, and magnetically stir at room temperature for five minutes to obtain the mixed solution B.
[0027] Transfer the mixed solution B to a 30mL stainless steel reactor lined with polytetrafluoroethylene, statically crystallize at a constant temperature of 180°C for 18h, cool to room temperature naturally, and then wash, centrifuge and dry with distilled water and absolute ethanol in sequence to obtain CaF 2 powder.
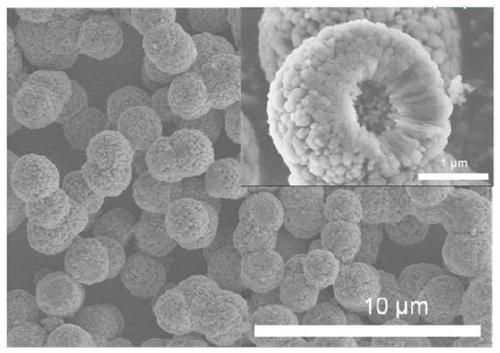
[0028] The synt...
Embodiment 2
[0032] Preparation of mixed solution A: Using F127 as surfactant, according to the molar ratio of F127 and deionized water of 0.0001, add 1.209g of F127 into 15ml of deionized water, and magnetically stir for 1 hour at room temperature to prepare mixed solution A.
[0033] Preparation of mixed solution B: F127:Ca(NO 3 ) 2 :Na 3 C 6 h 5 o 7 :NaBF 4 :H 2 O=0.093:1:0.5:2:838, 0.236g Ca(NO 3 ) 2 4H 2 O, 0.147g Na 3 C 6 h 5 o 7 2H 2 O and 0.219 g NaBF 4 Sequentially added to the mixed solution A every five minutes, and magnetically stirred at room temperature for five minutes to obtain the mixed solution B.
[0034]Transfer the mixed solution B to a 30mL stainless steel reactor lined with polytetrafluoroethylene, statically crystallize at a constant temperature of 150°C for 18h, cool to room temperature naturally, then wash, centrifuge and dry with distilled water and absolute ethanol in sequence to obtain CaF 2 powder.
[0035] XRD characterization results such as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com