Monoclonal antibody resisting skeletal muscle troponin I and application of monoclonal antibody
A monoclonal antibody, troponin technology, applied in the fields of immunology and food safety analysis, can solve the problems of loss of antigenicity and water solubility, protein denaturation, difficult immunological methods, etc., to achieve strong controllability and repeatability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment one The preparation of bovine skeletal muscle troponin I antigen of the present invention
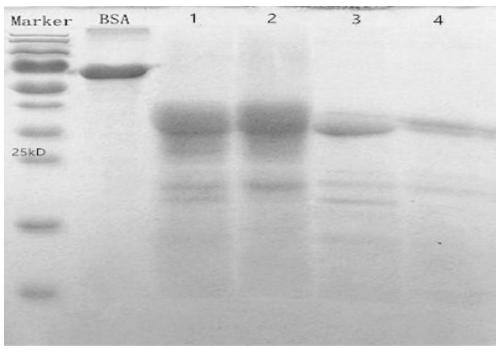
[0038]Take bovine skeletal muscle to remove fat and connective tissue, grind and mix evenly, weigh 40g and add 0.15M NaCL solution (1:2w / v); after further mixing, ultrasonically extract for 5min (50W, 20KHz), and then heat with boiling water After 20 minutes, centrifuge at 2000g for 30 minutes; remove the precipitate, take half of the supernatant and filter it as treatment solution 1. The other half of the supernatant was subjected to high pressure at 121°C for 30min, centrifuged at 5000g for 30min, filtered with Whatman No. 1 filter paper, added 90% ethanol (1:3.74v / v) to the filtrate, and the mixture was centrifuged at 7000g for 20min, and the precipitate was dried at 37°C , after reconstitution with physiological saline, it becomes the treatment solution 2. After treatment solution 1 and treatment solution 2 were identified by SDS-PAGE electrophoresis, the identifi...
Embodiment 2
[0039] Embodiment two The preparation of bovine skeletal muscle troponin I monoclonal antibody of the present invention
[0040] (1) Animal immunization: immunize 6-8 week-old female Balb / c mice with the prepared immune antigen, and immunize once every 2 weeks. rate, select the mouse with the best immune result to prepare for fusion;
[0041] Table 1 Immunization flow chart
[0042]
[0043] (2) Cell fusion: the fused mice were bleed from the eyeballs, and the serum was used as a positive control. After the neck was killed, the spleen was taken out under aseptic conditions to prepare spleen cells, which were fused with SP2 / 0 cells by PEG at a ratio of 5:1. The fused cell suspension was added to a 96-well plate with feeder cells, and placed in a 37°C, 5% CO 2 cultivated in an incubator;
[0044] (3) Screening of positive hybridoma cell lines: the fused cells were checked for contamination the next day, and replaced with HT medium on the 10th day after fusion. 2-3 days af...
Embodiment 3
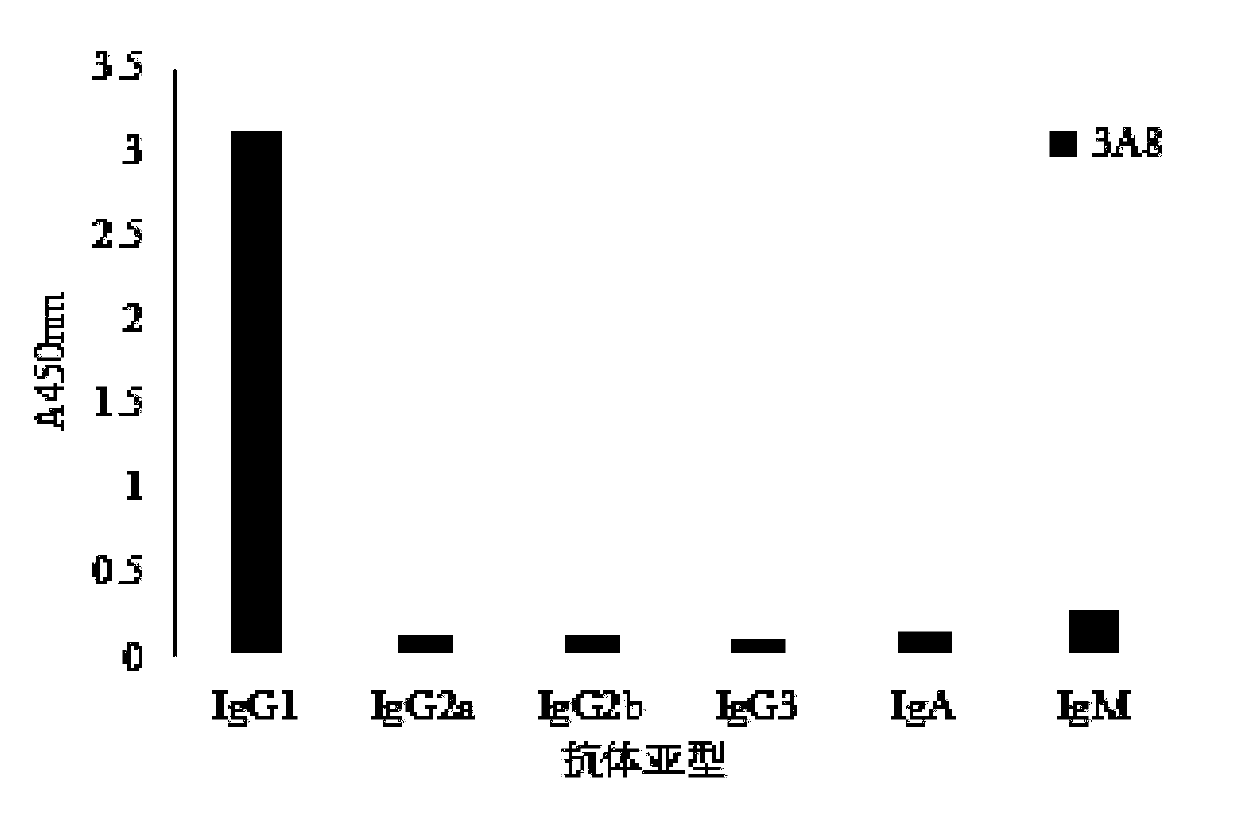
[0046] Embodiment three Characteristic identification of bovine skeletal muscle troponin I monoclonal antibody of the present invention
[0047] (1) Titer determination adopts indirect ELISA method, and the specific steps are as follows:
[0048] Coating: Dilute the coating material with carbonate buffer to a concentration of 5 μg / mL, 100 μL / well of a 96-well microtiter plate, overnight at 4°C;
[0049] Washing: return the coated plate to room temperature, pour off the coating solution, add 300 μL of washing solution to each well, let stand for 1 min each time, wash 3 times, and pat dry for the last time;
[0050] Blocking: Add 200 μL of washing solution containing 10% calf serum to each well, 37°C for 1 hour; pour off the blocking solution, wash 3 times, and pat dry;
[0051] Add the primary antibody: use the washing solution to dilute the monoclonal antibody at 1:2000 times, add 100 μL to each well, and set a blank control well (PBS) and a negative control (negative serum),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com