Formoterol, pharmaceutically acceptable salt thereof and preparation method of intermediate
A formoterol and intermediate technology, applied in the field of pharmaceutical synthesis, can solve the problems of incomplete crystallization, high temperature, affecting the yield of the compound of formula X, etc., and achieve the effects of reducing production costs and short routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
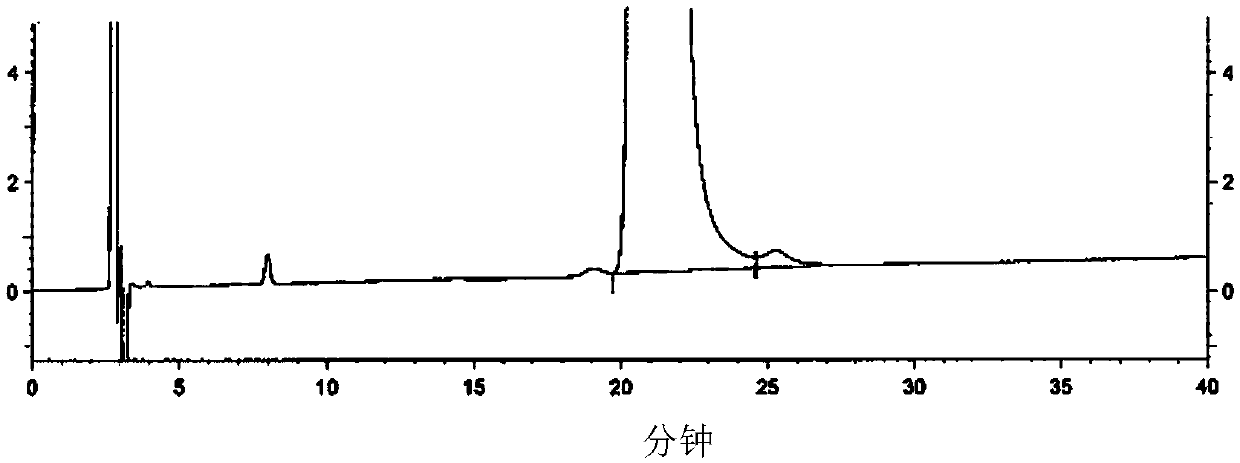
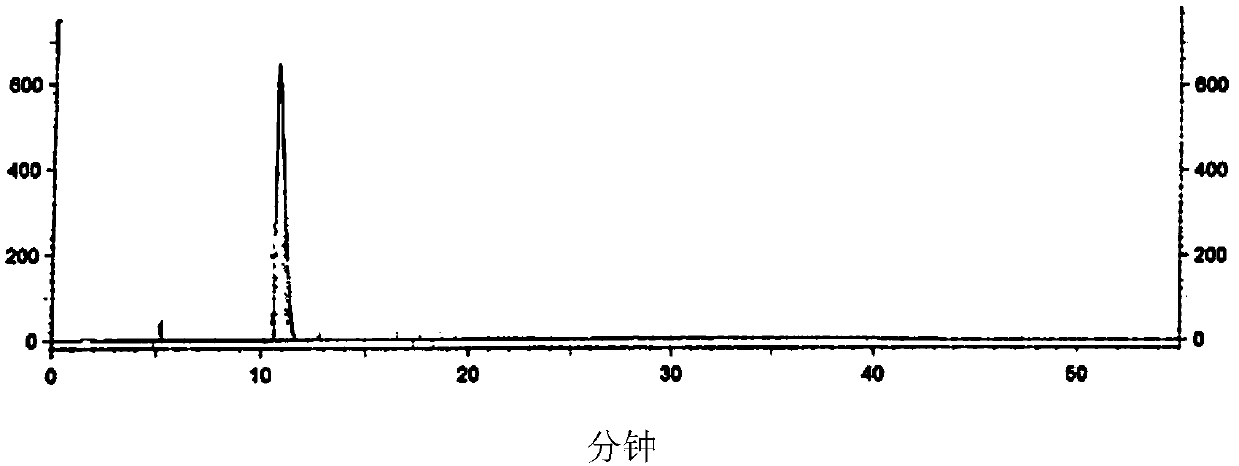
Image
Examples
Embodiment 1
[0092] 1) Add the compound of formula II (178.1g) and the compound of formula III (161.7g) into the reaction flask, stir, heat to an internal temperature of 90-100°C, and react for 2-3h; when the internal temperature is 120-130°C, react for 2-3h To complete (HPLC detection, calculated by the normalization method with the peak area, if the compound of formula III in the test sample is less than 10%, it can be considered that the reaction is complete), obtain 320.5g of the compound of formula IV, after cooling to 70 ± 5 ° C, add Water ethanol (220mL) was dissolved for the next reaction.
[0093]
[0094] 2) Add the compound of formula IV dissolved in absolute ethanol (220mL) and absolute ethanol (1280mL) into the reaction flask, stir, add reduced iron powder (199.7g), wash the residue with absolute ethanol (1000mL), and then add chloride Ammonium (191.3g) and water (440mL), heated to an internal temperature of 75-85°C, reacted for 2-3h to complete (HPLC detection, calculated ...
Embodiment 2
[0108] 1) Add V(A) compound (280g) and ethyl acetate (500mL) obtained in step 2) of Example 1 into the reaction flask, stir and dissolve, then add ethyl acetate (340mL), stir, and heat to internal temperature At 65-70°C, add fumaric acid (32.7g) and ethanol (210mL) until the sticky matter is completely dissolved, then cool down to 40°C at a rate of 10°C per hour, then cool down to 30-35°C in a water bath, and stir 1-2h, cool down to internal temperature 0-10°C, stir and crystallize for 1-2h, filter to obtain solid, then wash with ethyl acetate until the filtrate is pale yellow, then vacuum dry at 50±5°C for 4- After 6h, 190g of light yellow solid was obtained.
[0109] Add the light yellow solid (130g) obtained in the previous step, ethyl acetate (650mL) and 15% aqueous sodium carbonate solution (650mL) into the reaction flask, heat to an internal temperature of 30-40°C, a large amount of gas will be generated, stir for 4-6h, until The solid disappeared completely, left to st...
Embodiment 3
[0114] 1) high-purity formula V compound (65g) and ethyl acetate (300mL) are added to reaction flask, under N 2 The reaction solution was cooled to 0-10°C under the condition, and then dicyclohexylcarbodiimide (DCC) (62.3g) and ethyl acetate (25mL) were added, and slowly added dropwise at an internal temperature of 0-10°C without Aqueous formic acid (12.0g), after the dropwise addition, keep the internal temperature at 0-10°C, and react for 1-2h until the reaction is complete (detected by HPLC, calculated by the normalization method based on the peak area, the test product has the formula V compound is less than 0.50%, the reaction can be considered complete.), and then close the cooling equipment, add 10% sodium carbonate aqueous solution (275mL), heat up to 20-30 ° C, stir for 0.5-1.0h, separate the organic layer, and the aqueous layer with acetic acid Ethyl ester (275mL) was extracted, then combined with ethyl acetate layer, stirred and washed with 10% sodium carbonate aque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com