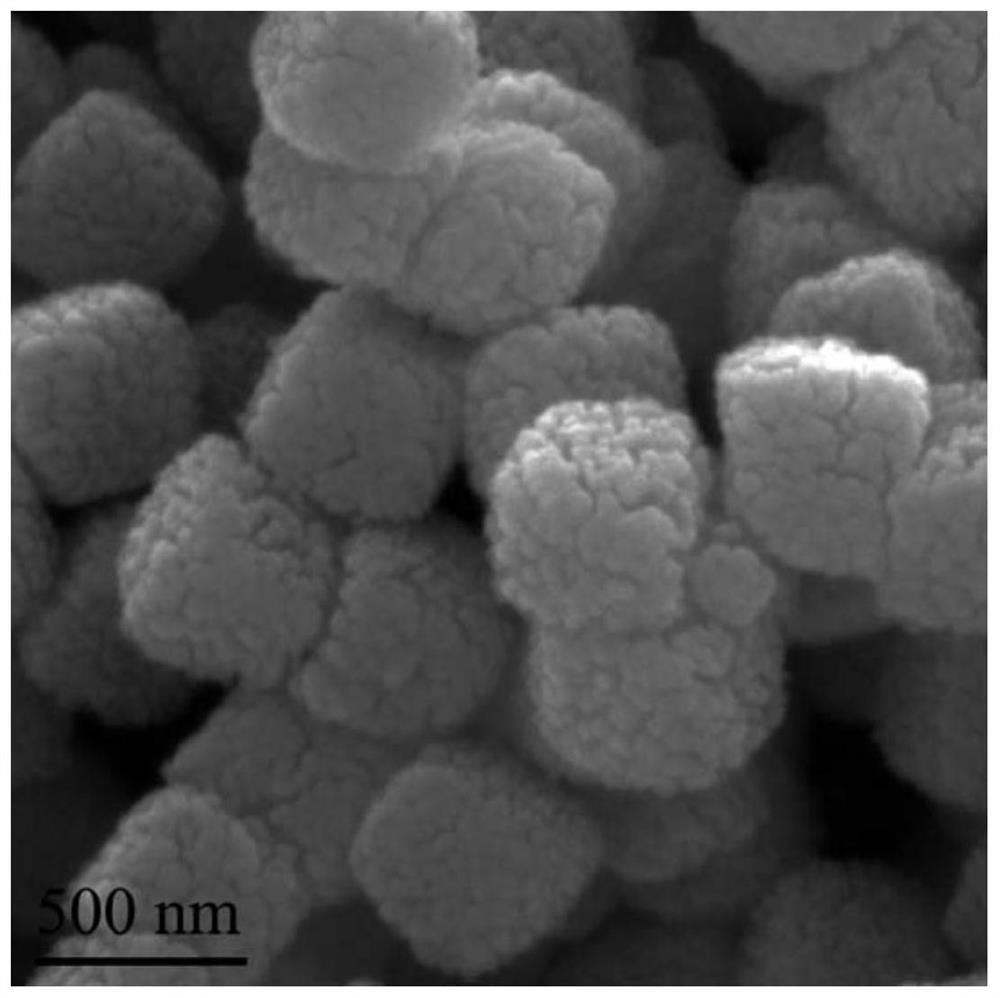
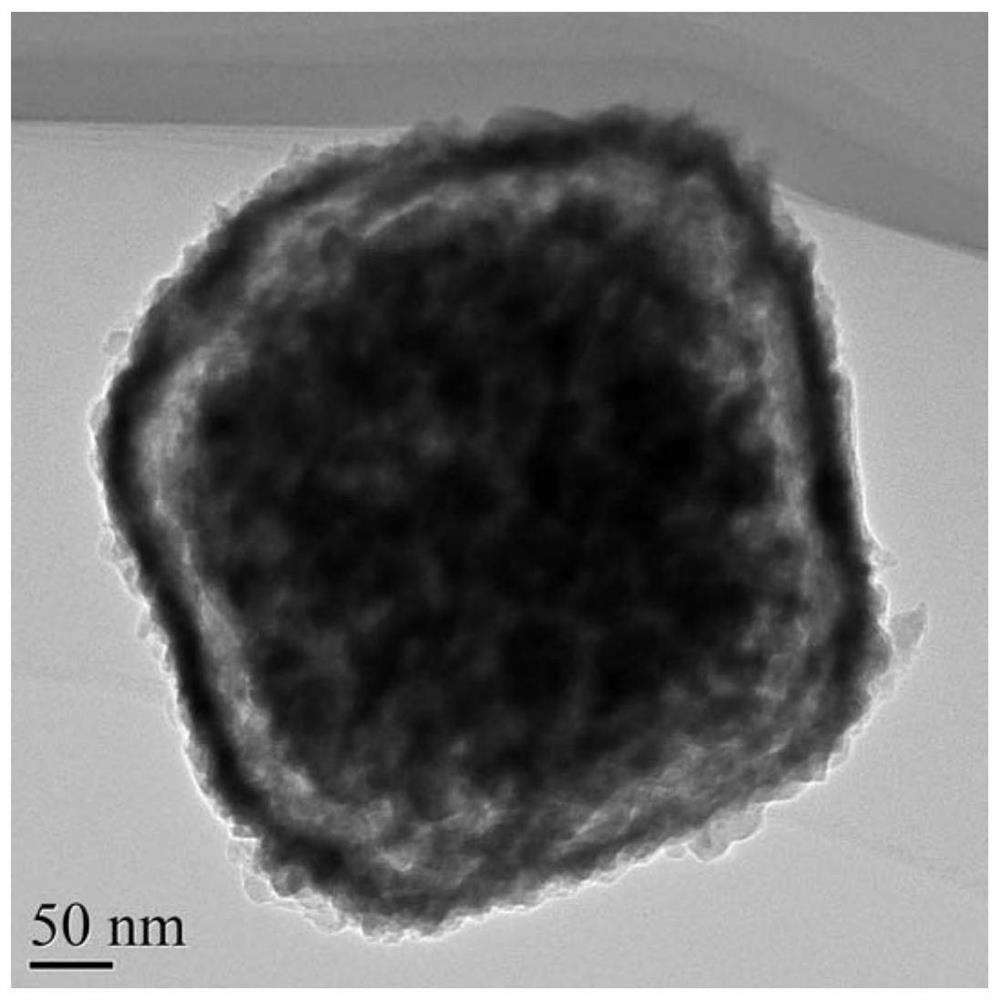
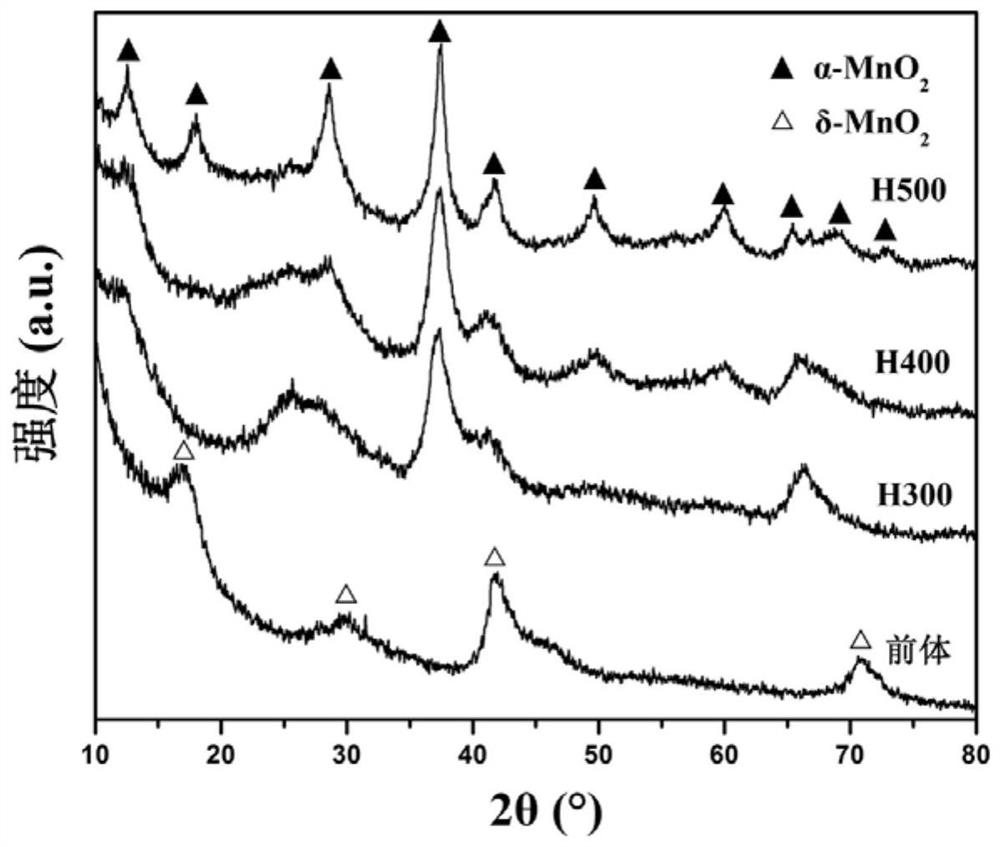
A kind of egg yolk-shell structure manganese potassium ore type manganese dioxide catalyst and its preparation method and application
A technology of manganese dioxide and shell structure, which is applied in the field of egg yolk-shell structure manganese potassium ore-type manganese dioxide catalyst and its preparation, can solve the problems of long synthesis cycle and high device pressure, and achieve low cost, high catalytic activity, and process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Dissolve 1.58g of ammonium bicarbonate in 25mL of deionized water, add 500mL of cyclohexane, 25mL of n-butanol, and 20g of cetyltrimethylammonium bromide, and continue stirring until the solution is clear.
[0024] After dissolving 1.69g of manganese sulfate in 25mL of water, add it dropwise to the above mixture, and keep stirring for 20min.
[0025] The solution was centrifuged at 9000r for 3~5min, and the solid residue was washed with ethanol and water alternately for 3 times.
[0026] Put it into a vacuum oven at 90°C for 8 hours to obtain manganese carbonate powder.
[0027] Dissolve 1 g of the dried manganese carbonate powder in 272 mL of deionized water, add 1.375 g of potassium permanganate, ultrasonicate for 2 min, and stir for 20 min.
[0028] The obtained solution was centrifuged at 9000r for 3~5min, and the solid residue was washed with ethanol and water alternately for 3 times.
[0029] Put it into an oven at 100°C and dry for 8 hours to obtain a precursor...
Embodiment 2
[0032] Dissolve 1.58g of ammonium bicarbonate in 25mL of deionized water, add 500mL of cyclohexane, 25mL of n-butanol, and 20g of cetyltrimethylammonium bromide, and continue stirring until the solution is clear.
[0033] After dissolving 1.69g of manganese sulfate in 25mL of water, add it dropwise to the above mixture, and keep stirring for 20min.
[0034] The solution was centrifuged at 9000r for 3~5min, and the solid residue was washed with ethanol and water alternately for 3 times.
[0035] Put it into a vacuum oven at 90°C for 8 hours to obtain manganese carbonate powder.
[0036] Dissolve 1 g of the dried manganese carbonate powder in 272 mL of deionized water, add 1.375 g of potassium permanganate, ultrasonicate for 2 min, and stir for 20 min.
[0037] The obtained solution was centrifuged at 9000r for 3~5min, and the solid residue was washed with ethanol and water alternately for 3 times.
[0038] Put it into an oven at 100°C and dry for 8 hours to obtain a precursor...
Embodiment 3
[0041] Dissolve 1.58g of ammonium bicarbonate in 25mL of deionized water, add 500mL of cyclohexane, 25mL of n-butanol, and 20g of cetyltrimethylammonium bromide, and continue stirring until the solution is clear.
[0042] After dissolving 1.69g of manganese sulfate in 25mL of water, add it dropwise to the above mixture, and keep stirring for 20min.
[0043] The solution was centrifuged at 9000r for 3~5min, and the solid residue was washed with ethanol and water alternately for 3 times.
[0044] Put it into a vacuum oven at 90°C for 8 hours to obtain manganese carbonate powder.
[0045] Dissolve 1 g of the dried manganese carbonate powder in 272 mL of deionized water, add 1.375 g of potassium permanganate, ultrasonicate for 2 min, and stir for 20 min.
[0046] The obtained solution was centrifuged at 9000r for 3~5min, and the solid residue was washed with ethanol and water alternately for 3 times.
[0047] Put it into an oven at 100°C and dry for 8 hours to obtain a precursor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com