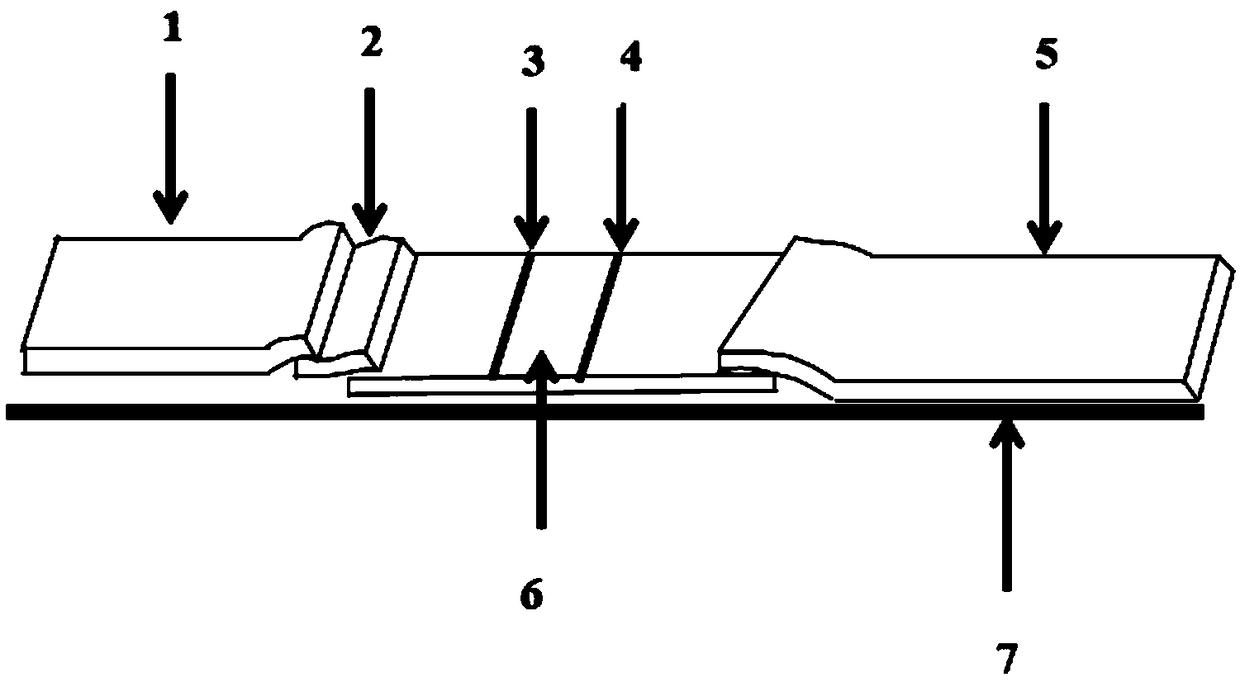
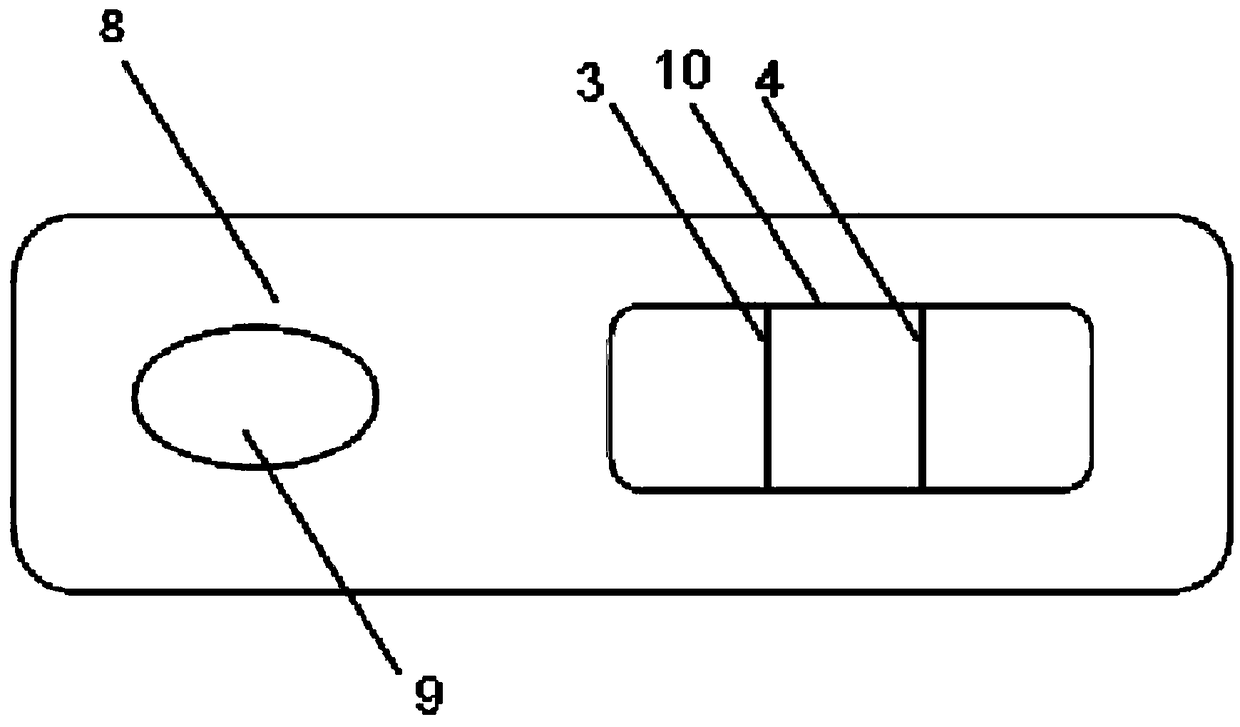
Preparation method of NSE test strip, test strip, test card and NSE test kit
A technology for detecting test paper and test strips, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve the effects of shortened detection time, wide application range, and improved detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of NSE detection test strip, detection card and kit set
[0030] 1.1 Preparation of conjugation pads: (1) Preparation of NSE antibody-fluorescent microsphere complex: take 10 μL of Eu-carboxyl fluorescent microspheres (suspension, particle size 200 nm, concentration 112 ueq / g) and add 400 μL of 0.05M boric acid buffer at pH=7.6 solution, mix well; add 40 μL 1mg / ml EDC activator and 10 μL 1mg / ml NHS solution, shake and activate on a constant temperature oscillator for 20 minutes; then centrifuge at 5000 rpm for 20 minutes, discard the supernatant, and add 500 μL 0.05M pH=7.6 boric acid buffer Reconstitute the precipitate in the solution, vortex to mix, add 40ugNSE antibody, vortex to mix, place in a shaker for low-speed oscillating coupling for 2h; centrifuge at 15000rpm for 15min, discard the supernatant, add 1500μL of reconstitution solution to redissolve, and then use 10μL of blocking solution ( 20%BSA) for blocking 30min, the complex solutio...
Embodiment 2
[0051] Embodiment two, control experiment
[0052] 1. The rest of the conditions remain the same, replace the complex solution with 0.01M Tris-Hcl PH = 8.0, 0.1% Tween-20, 1% BSA, 0.1% PVP, 0.05% PEG-200, 0.5% glycine and 3% trehalose solution (the concentration of each of the above components is the final concentration);
[0053] The treatment solution was replaced with: 0.01M Tris-Hcl PH=8.0, 0.1% T-20, 0.1% BSA and 5% sucrose (the concentrations of the above components are the final concentrations);
[0054] Others were all prepared test strips according to the method of Example 1, and further made into test cards, and carried out sample determination.
[0055] 2. Draw the standard curve: (1) Sample acquisition: The blood sample is provided by the hospital and the quality control value is determined. On this basis, the normal saline is used for gradient dilution. The assigned units are: 0, 0.39, 0.78, 1.56 , 3.125, 6.25, 12.5, 25, 50, 100, 200, 300, 400, 500; the real value...
Embodiment 3
[0061] Embodiment three, the detection effect verification of the test strip / detection card that embodiment one makes:
[0062] 1. measure the detection limit of the detection card / test strip qualitative detection that the present invention (embodiment one) makes
[0063] (1) Sample processing: collect blood with the sample collection device provided in this kit.
[0064] (2) Preparation of test card or test strip: place the prepared test card / test strip at room temperature for 15 minutes before use.
[0065] Dilute the NSE standard to 2ng / mL, 4ng / mL, 8ng / mL, 16ng / mL, 32ng / mL, 64ng / mL, 128ng / mL, then add sample saline to the standard for dilution (per drop of sample Add 3mL to simulate the actual detection situation), after dilution, add 65μL to the sample pad for each detection for detection.
[0066] After adding the sample for 5 minutes, read under the ultraviolet light. The results are shown in Table 3:
[0067] Table 3 NSE qualitative detection detection limit determi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com