Preparation method and application of third generation LMP2A CAR-T cells
A cell and extracellular region technology, applied in the field of preparation of third-generation LMP2ACAR-T cells, can solve problems such as LMP2ACAR-T cells without third-generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
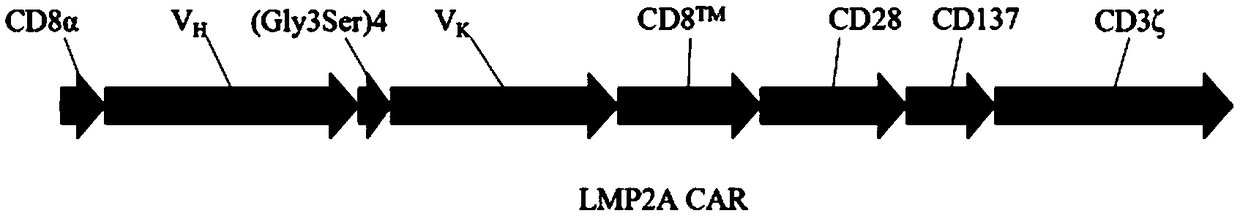
[0099] Example 1 Construction of lentiviral expression vector for anti-LMP2A CAR
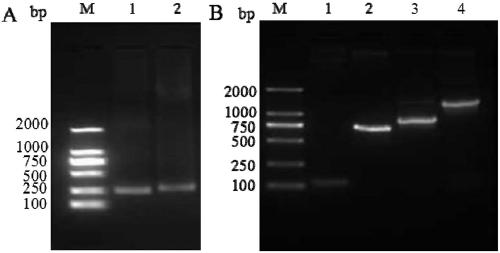
[0100] 1.1 Design and synthesis of LMP2A scFv primers targeting the extracellular region of the chimeric antigen receptor LMP2A CAR targeting LMP2A
[0101] The fully human anti-LMP2A antibody Fab constructed in the laboratory was analyzed with the biological software DNAstar 8.0, and the light chain variable region (Vκ) and heavy chain variable region (Vκ) were designed to be amplified. H ) primers were optimized, and the primers were finally synthesized by Beijing Qingke Xinye Biotechnology Co., Ltd. LMP1scFv heavy and light chain variable region primer sequences are as follows:
[0102]
[0103] The direction of the above primers are all 5'-3'
[0104] 1.2 Synthesis and amplification of the heavy and light chain variable regions of the LMP2A scFv segment of the extracellular domain of the chimeric antigen receptor LMP2A CAR targeting LMP2A
[0105] 1) Reaction system (take 50ul system a...
Embodiment 2
[0189] Example 2 LMP2A CAR virus packaging
[0190] 1) Collect X-293T cells, inoculate them on a 10cm culture dish and continue culturing. When the cell confluence reaches 70%, replace with fresh incomplete medium 30 minutes before virus packaging; 2) 1.5ml centrifuge tube A: 470ul Opti-MEM+30ul PEI , after resuspension, incubate at room temperature for 5min; 1.5ml centrifuge tube B: 3.3ug psPAX2+3.3ug pMD2.G+3.3ug LMP2A CAR, add Opti-MEM to make the volume to 250ul; 3) Drop the reagents in tube A into tube B , mix gently, and place at room temperature for 15 minutes; 4) Add the mixture dropwise to X-293T cells, shake the culture dish gently to make the mixture evenly distributed, and incubate in a 37°C incubator; 5) After 6-8 hours, replace with fresh Complete medium (1640+10% FBS); 6) Observe the transfection efficiency under a fluorescent microscope after 24 hours; 7) Collect the supernatant after 48 hours, if the cells adhere well, replace with fresh incomplete medium (164...
Embodiment 3
[0191] Example 3 Concentration of LMP2A CAR virus
[0192] method one:
[0193] 1) After the supernatant collected in Example 2 was filtered through a 0.45um membrane filter, it was transferred to a 100kD ultrafiltration tube and concentrated to 1 / 3 volume
[0194] 2) The concentrated solution is filtered through a 0.22um filter membrane, and the filtrate is collected and stored at -80°C
[0195] Method Two:
[0196] 1) Dissolve 8.76g NaCl and 50g PEG-8000 in 200ml double distilled water respectively, and autoclave to prepare 5×PEG-8000 NaCl solution; 2) The virus supernatant collected in Example 2 was mixed with 5 Mix ×PEG-8000NaCl, shake once every 20-30min, 5 times in total, overnight at 4°C; 3), centrifuge at 6000rpm for 20min, remove the supernatant; 4) collect the precipitate, resuspend in medium, and freeze at -80°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com