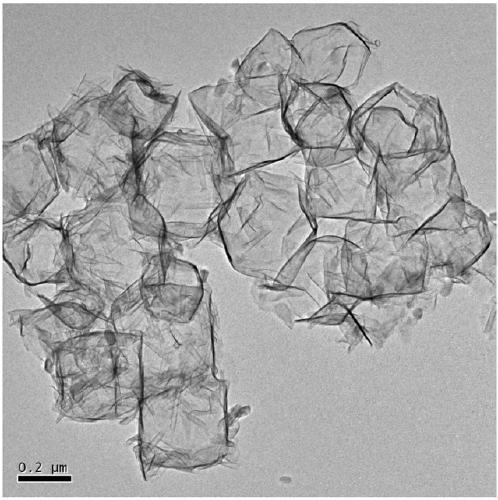
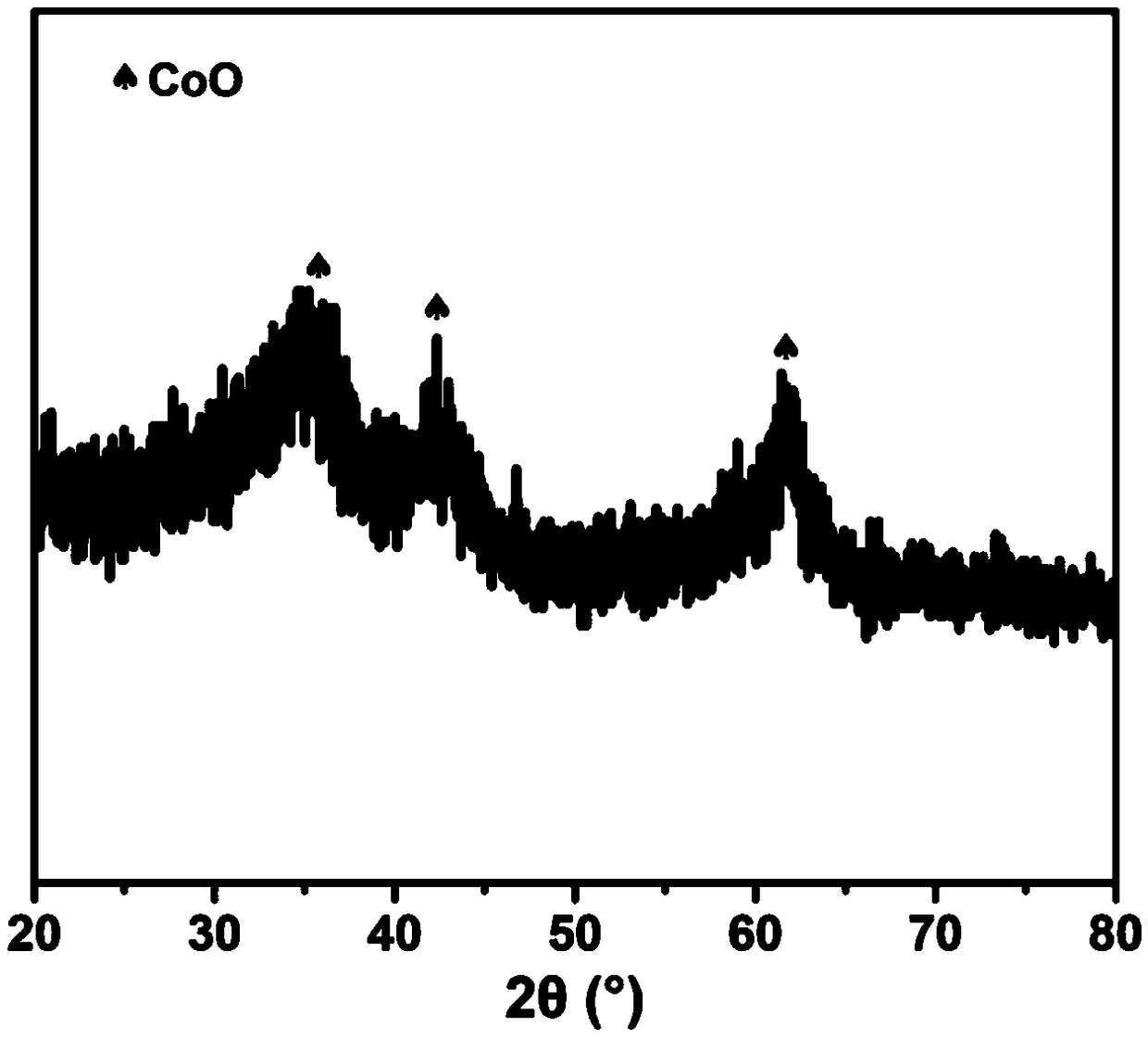
Cobalt iron oxide hollow nano-cage material, preparation method and uses thereof
A cobalt-iron oxide and nanocage technology, applied in the field of electrocatalytic materials, can solve the problems of high price, poor activity and stability, etc., and achieve the effects of convenient operation, simple equipment and simple synthesis path
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Weigh 1.45g of cobalt nitrate hexahydrate and 3.24g of dimethylimidazole and dissolve them in 100mL of methanol, pour the former into the latter under vigorous stirring, stop stirring after 10min, and let stand at room temperature for 24h , after centrifugation, washed twice with methanol and once with ethanol to obtain ZIF-67. The obtained ZIF-67 was dispersed in 100 mL of ethanol to form a dispersion liquid.
[0033] (2) Weigh 61.5mg of ferrous acetate tetrahydrate (the molar weight of ferrous acetate tetrahydrate is 0.25mmol) and 56.1mg of hexamethylenetetramine, dissolve them in 8mL of ultrapure water, and add them to 20mL under stirring. (1) The obtained ZIF-67 dispersion liquid was heated to reflux for 30 minutes, then centrifuged, washed twice with ethanol, and dried overnight at 60° C. to obtain a solid.
[0034] (3) Grind the dry solid obtained in step (2) into powder and evenly spread it on the bottom of the silica ark and put it into the middle of the tu...
Embodiment 2
[0038] (1) The preparation of the ZIF-67 dispersion is the same as in Example 1.
[0039] (2) Weigh 24.6 mg of ferrous acetate tetrahydrate (the molar weight of ferrous acetate tetrahydrate is 0.1 mmol) and 22.4 mg of hexamethylenetetramine and dissolve them in 8 mL of ultrapure water, and add 20 mL of ZIF- 67 ethanol solution, heated to reflux for 30 min, then centrifuged, washed twice with ethanol, and dried overnight at 60°C.
[0040] (3) Grind the dry product obtained in step (2) into powder and evenly spread it on the bottom of the silica ark and put it into the middle of the tube-type atmosphere furnace. 2 / Ar (V / V=1 / 9) mixed atmosphere at 5°C min -1 After heating up to 320° C., calcining for 2 hours to obtain the cobalt-iron oxide hollow nanocage material.
[0041] The preparation of the working electrode and the test of its oxygen evolution catalytic performance are the same as in Example 1. The result is: when the current density is 10mA cm -2 , the reaction overpot...
Embodiment 3
[0043] (1) The preparation of the ZIF-67 dispersion is the same as in Example 1.
[0044] (2) Weigh 98.4 mg of ferrous acetate tetrahydrate (the molar weight of ferrous acetate tetrahydrate is 0.4 mmol) and 89.6 mg of hexamethylenetetramine and dissolve them in 8 mL of ultrapure water, and add 20 mL of ZIF- 67 ethanol solution, heated to reflux for 30 min, then centrifuged, washed twice with ethanol, and dried overnight at 60°C.
[0045] (3) Grind the dry product obtained in step (2) into powder and evenly spread it on the bottom of the silica ark and put it into the middle of the tube-type atmosphere furnace. 2 / Ar (V / V=1 / 9) mixed atmosphere at 5°C min -1 After heating up to 320° C., calcining for 2 hours to obtain the cobalt-iron oxide hollow nanocage material.
[0046] The preparation of the working electrode and the test of its oxygen evolution catalytic performance are the same as in Example 1. The result is: when the current density is 10mA cm -2 , the reaction overp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com