Method of purifying HBc-VLPs (hepatitis B virus core virus-like particles) or HBc-VLPs derivatives
A technology of derivatives and purity, applied in the field of purifying HBc-VLPs or HBc-VLPs derivatives, can solve the problems of low purity, low yield, and many steps of HBc-VLPs, achieving good repeatability, reducing production costs, time saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] In this example, the bacteria solution containing HBc-VLPs was prepared through the following steps
[0074] HBc-VLPs质粒构建与转化:乙肝核心抗原的核苷酸序列,SEQ ID NO.1:ATGGACATTGACCCTTATAAAGAATTTGGAGCTACTGTGGAGTTACTCTCGTTTTTGCCTTCTGACTTCTTTCCTTCCGTCAGAGATCTCCTAGACACCGCCTCAGCTCTGTATCGAGAAGCCTTAGAGTCTCCTGAGCATTGCTCACCTCACCATACTGCACTCAGGCAAGCCATTCTCTGCTGGGGGGAATTGATGACTCTAGCTACCTGGGTGGGTAATAATTTGGAAGATCCAGCATCCAGGGATCTAGTAGTCAATTATGTTAATACTAACATGGGTTTAAAGATCAGGCAACTATTGTGGTTTCATATATCTTGCCTTACTTTTGGAAGAGAGACTGTACTTGAATATTTGGTCTCTTTCGGAGTGTGGATTCGCACTCCTCCAGCCTATAGACCACCAAATGCCCCTATCTTATCAACACTTCCGGAAACTACTGTTGTTAGACGACGGGACCGAGGCAGGTCCCCTAGAAGAAGAACTCCCTCGCCTCGCAGACGCAGATCTCAATCGCCGCGTCGCAGAAGATCTCAATCTCGGGAATCTCAATGTTAG.
[0075] Its amino acid sequence SEQ ID NO.2: MDIDPYKEFGATVELLSFLPSDFFFPSVRDLLDTASALYREALESPEHCSPHHTALRQAILCWGELMTLATWVGNNLEDPASRDLVVNYVNTNMGLKIRQLLWFHISCLTFGRETVLEYLVSFGVWIRTPPAYRPPNAPILSTLPETTVVRRRDRGRSPRRRTPSQPRRRRSQSPRRRSQSRES
[0076] Through PCR amplification, the ampl...
Embodiment 2
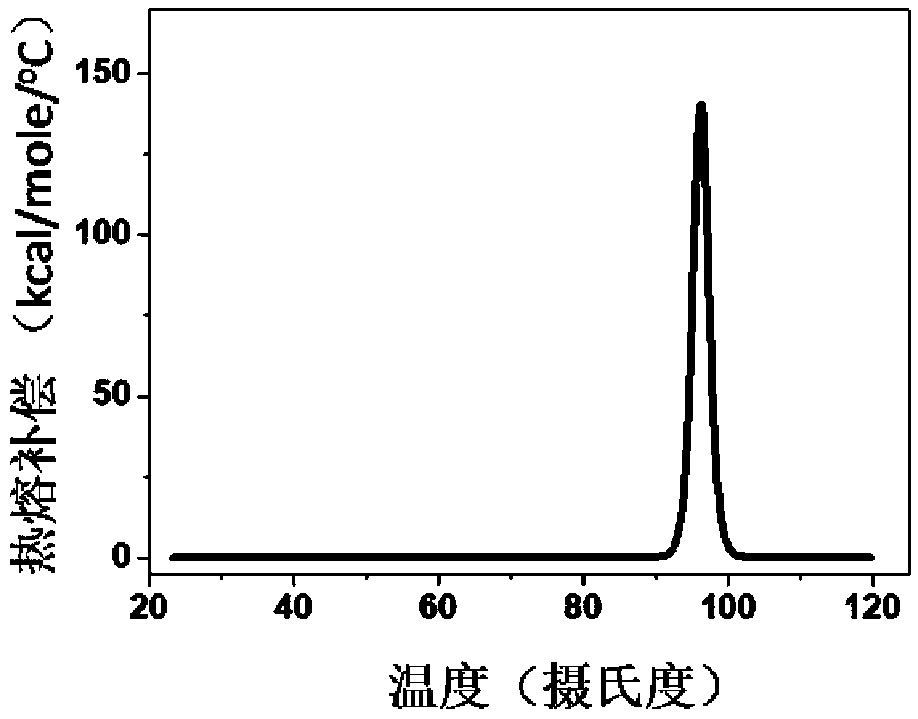
[0083] In this embodiment, the thermal stability detection of HBc-VLPs and the determination of the heating temperature are carried out
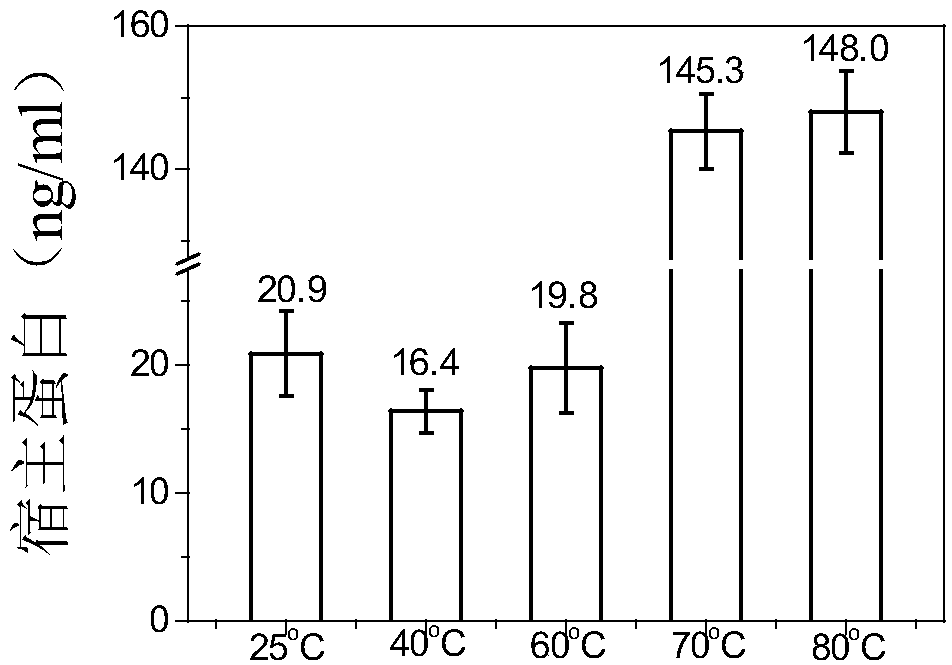
[0084] Using DSC technology to detect the thermal stability of HBc-VLPs, such as figure 1 As shown, its denaturation temperature is 96°C. In order to make full use of the advantage of the heat resistance of HBc-VLPs, the effects of different heating temperatures and times on the removal rate of impurity proteins and the yield of target proteins were investigated. In the case of heating for 30 minutes, the heating temperature of the supernatant of cell disruption increased from When 25°C (room temperature) was increased to 80°C, the removal rate of impurity proteins increased gradually ( Figure 2A ). The heat-treated samples were purified by density gradient centrifugation to obtain pure HBc-VLPs, and then the particles were denatured with denaturants such as urea or guanidine hydrochloride to completely crack the virus-like particles and ...
Embodiment 3
[0087] In this embodiment, the secondary gradient heating is carried out, and the high performance liquid phase detection of the bacterial liquid is carried out
[0088] The broken supernatant of bacteria after heat treatment at 60° C. for 30 minutes was centrifuged at 4° C. for 15 minutes, then heat-treated at 70° C. for 30 minutes, and centrifuged at 4° C. for 20 minutes. TSK5000 high performance liquid phase size exclusion chromatography was used to analyze the protein changes in the supernatant during the two gradient heating processes. The chromatographic analysis conditions were: mobile phase: 50mM PB buffer with pH 6.8; flow rate: 0.5mL / min; : 100 μL.
[0089] The result is as image 3 As shown, the peak at the retention volume of 5.6 mL is HBc-VLPs. Comparing the broken cell supernatant before and after heat treatment at 60°C, it was found that the protein content decreased significantly after the retention volume was 11.8 mL. After further heating at 70°C for 30min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com