Reference product for non-invasive prenatal detection of fetal aneuploid chromosomes
A technology for aneuploidy and prenatal detection, applied in the determination/inspection of microorganisms, DNA/RNA fragments, recombinant DNA technology, etc. Easy quality control, important application prospects, easy to prepare results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 prepares positive reference substance by the genomic DNA of tissue and blood source
[0023] Sample selection: Sample 1 is a placental tissue sample of a T21 trisomy (Down syndrome) positive male fetus. The reason for selecting a male fetus is to facilitate the quantitative detection of the incorporation ratio of mixed nucleic acids; Sample 2 is a maternal sample, that is, the fetus Maternal peripheral blood, two samples genetically related, was used to simulate the composition of cell-free DNA derived from maternal peripheral blood. Sample 3 is a plasma sample of a pregnant woman, which is used to provide plasma cell-free DNA.
[0024] DNA extraction: Genomic DNA of sample 1 (placental tissue sample) was extracted using a genome extraction kit (DP304, Tiangen Biochemical Technology Beijing Co., Ltd.) according to the instructions. For sample 2 (peripheral blood), red blood cells were first lysed, and then the genomic DNA was extracted from the isolated wh...
Embodiment 2
[0031] Example 2 Prepare positive reference substance by culturing the genomic DNA derived from cells
[0032] Sample selection: sample 4 is the T21 trisomy-positive cell line AG09394 (male), and sample 5 is the corresponding mother's cell line AG09387. Use RPMI 1640 medium, containing 2mM L-glutamine, 15% fetal bovine serum for cell culture, add 10-20ml medium (vertical placement culture) to T25 tissue culture flask, 37 ° C, CO2 concentration 5% incubator culture . Keep the cell concentration in the medium not lower than 200,000 cells / ml. Fresh medium should be replaced every 3-4 days. The upper limit of culture density is 1 million cells / ml, count the cells after culture, and then count 1×10 8 Cell Genome Extraction Kit (DP304, Tiangen Biochemical Technology Beijing Co., Ltd.) was used to extract the cellular genomic DNA of samples 4 and 5 according to the instructions.
[0033] DNA Fragmentation: Fragmentation of DNA and purification of magnetic beads according to the m...
Embodiment 3
[0037] Example 3 Detection of T21 Positive Reference Substances by Noninvasive Prenatal DNA Detection Reagents
[0038] In order to verify whether the prepared positive reference product for non-invasive prenatal detection of fetal aneuploid chromosomes can meet the needs of non-invasive prenatal detection, this experiment uses the non-invasive prenatal next-generation sequencing kit (Boao Bio) to test it. The test samples are plasma free DNA and two fragmented DNA mixtures in Example 1 and Example 2.
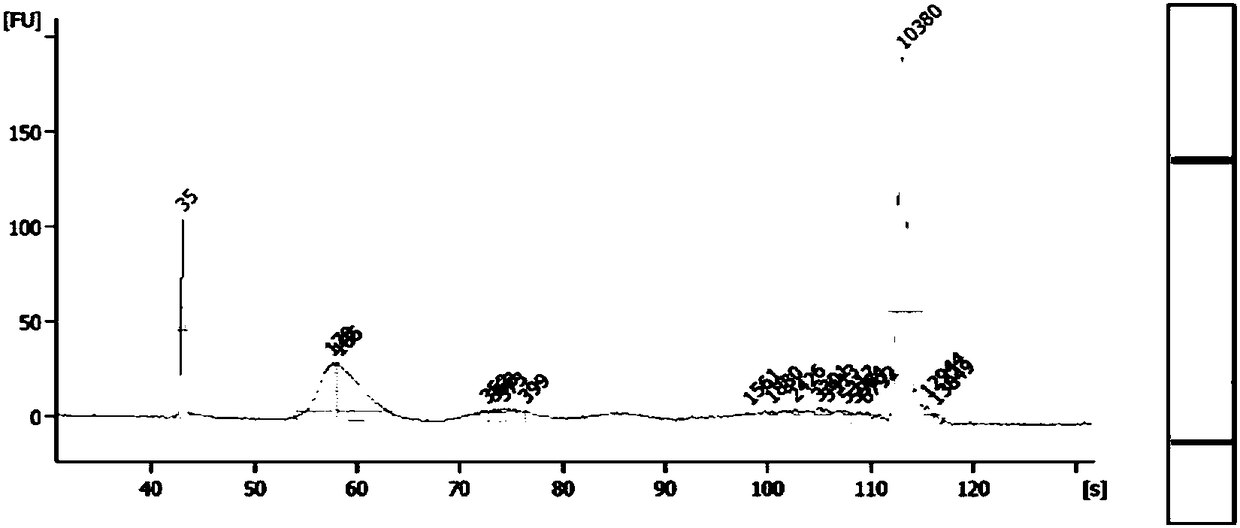
[0039] First, the library was constructed according to the instructions, and then the library concentration was tested by qPCR. The library concentration results are shown in Table 2. The data in Table 2 shows that there is no difference in the library concentration among the three samples, and the library construction results meet the test expectations.
[0040] Then the library was sequenced and analyzed, and the above three samples were sequenced according to the normal SOP ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com