Method for catalyzing conversion of fructose to 5-hydroxymethylfurfural by virtue of inorganic salt-gluconic acid system
A technology of hydroxymethylfurfural and gluconic acid, applied in the field of chemical reaction, can solve the problems of long reaction time, high reaction temperature, and low selectivity, and achieve the effects of reducing corrosion, high fructose conversion rate, and high HMF selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
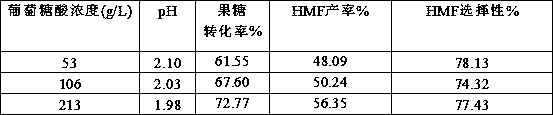
[0018] Embodiment 1 The influence of different gluconic acid concentration on reaction result
[0019] Weigh different amounts of gluconic acid and 1 g of fructose to 10 mL volumetric flasks to obtain mixed solutions of different concentrations of gluconic acid and 10% fructose. Take 1 mL of the mixed solution with different concentrations of gluconic acid as the catalyst in four 10 mL microwave tubes. React in a microwave reactor at 150°C for 10 minutes. Various products in the reaction solution were quantitatively analyzed by gas phase and liquid chromatography.
[0020] The aqueous detection method is: Agilent high performance liquid chromatography (HPLC 1260) equipped with Bio-Rad Aminex HPX-87H chromatographic column and dual detectors of ultraviolet and differential, mobile phase is 5mM dilute sulfuric acid, flow rate is 0.6mL / min, column temperature The temperature of the differential detector is 35 °C, the temperature of the differential detector is 35 °C, and the in...
Embodiment 2
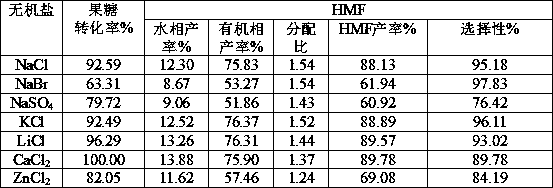
[0024] Example 2 The influence of salt effect on the pH change of gluconic acid solution
[0025] Weigh 10g of fructose and 21.78g of gluconic acid (50%wt gluconic acid solution) in a 100mL volumetric flask and dilute to 100mL with water to obtain a 10% fructose and 106g / L gluconic acid mixture. Weigh 1.000g NaCl, NaBr, KCl, LiCl, CaCl respectively 2 , ZnCl 2 Dissolve the different inorganic salts in a 10mL volumetric flask with the mixed solution, and dilute to 10mL. The measured pH is shown in Table 2:
[0026] Table 2 Effect of different inorganic salts on ionization degree of gluconic acid
[0027] salt type
[0028] From the data in Table 2, it can be seen that adding 10% inorganic salt can significantly increase the ionization degree of gluconic acid, thereby increasing the concentration of hydrogen ions in the system and decreasing the pH value. When a strong electrolyte (various inorganic salts) that does not have the same ions as the weak electrolyte is ...
Embodiment 3
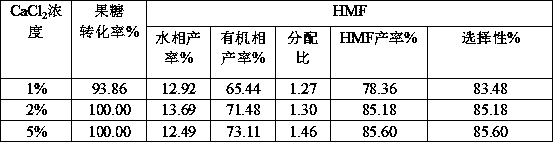
[0029] Example 3 Effects of different salt ions on gluconic acid-catalyzed dehydration of fructose
[0030] Weigh 10g fructose and 21.78g gluconic acid (50%wt gluconic acid solution) in a 100mL volumetric flask and dilute to 100mL with water to obtain 10% fructose and 108.9g / l gluconic acid mixed solution. Weigh 0.100g NaCl, NaBr, KCl, LiCl, CaCl 2 , ZnCl 2 Add 1mL of the mixed solution to ten 10mL microwave tubes respectively; then add 4mL of 2-butanone, and react in a microwave reactor at 150°C for 10min. The results are shown in Table 3:
[0031] Table 3 The promotion effect of different inorganic salts on the dehydration of fructose catalyzed by gluconic acid
[0032]
[0033] It can be seen from Table 2 that different inorganic salts have different effects on the ionization effect of gluconate hydrogen ions, while in Table 3 it is shown that the effects on the catalytic dehydration of fructose to prepare HMF are also different. Among them, calcium chloride has a be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com