Method of producing low carbon olefin through direct conversion of catalyst and synthesis gas
A catalyst and synthesis gas technology, which is applied in catalyst activation/preparation, catalyst, catalyst carrier and other directions, can solve the problems of reduced selectivity and low selectivity of low-carbon olefins in products, and achieves improved conversion rate, long catalyst life, and good application. Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] One, the preparation of catalyst A
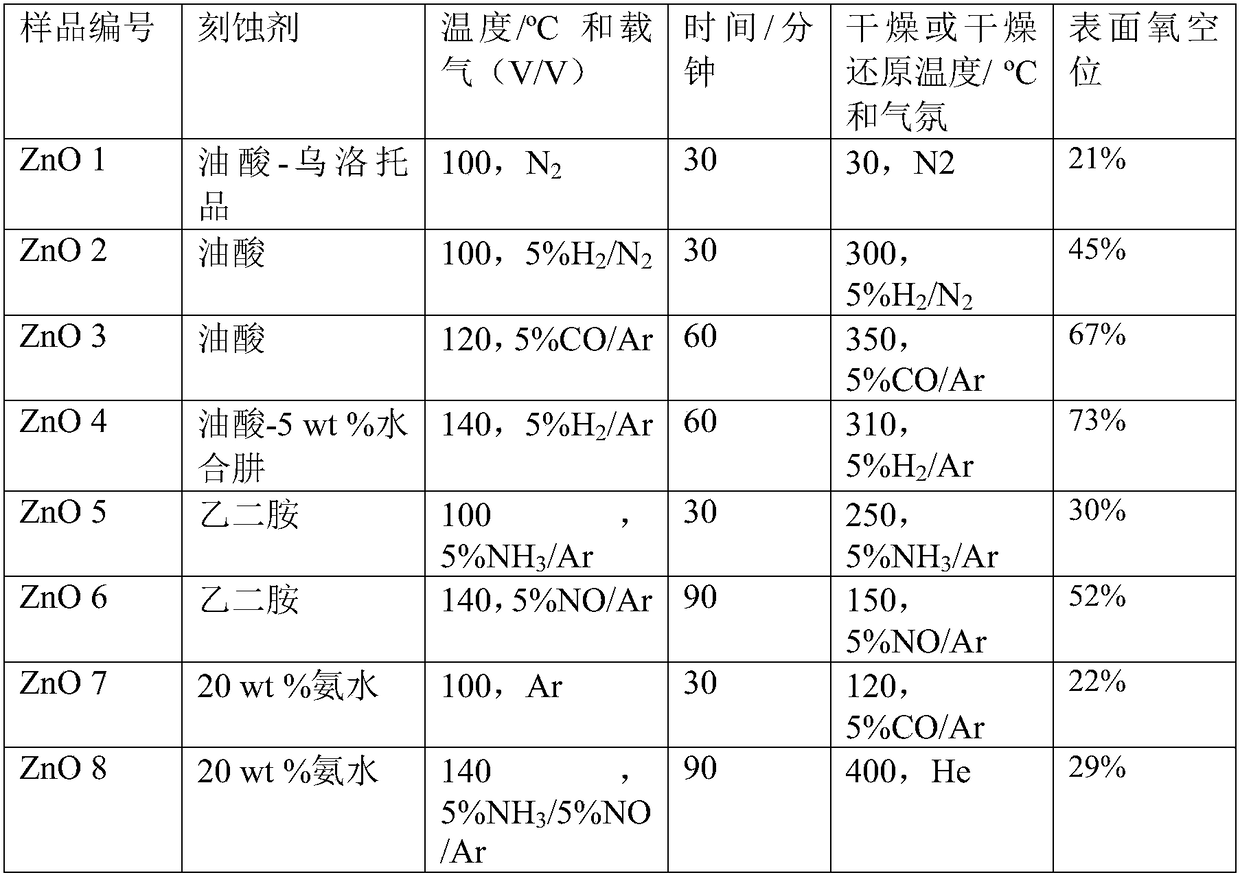
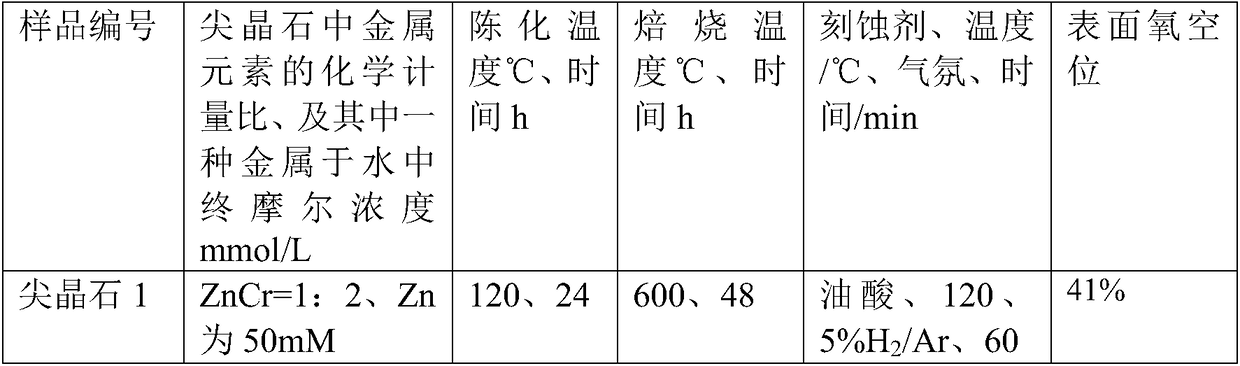
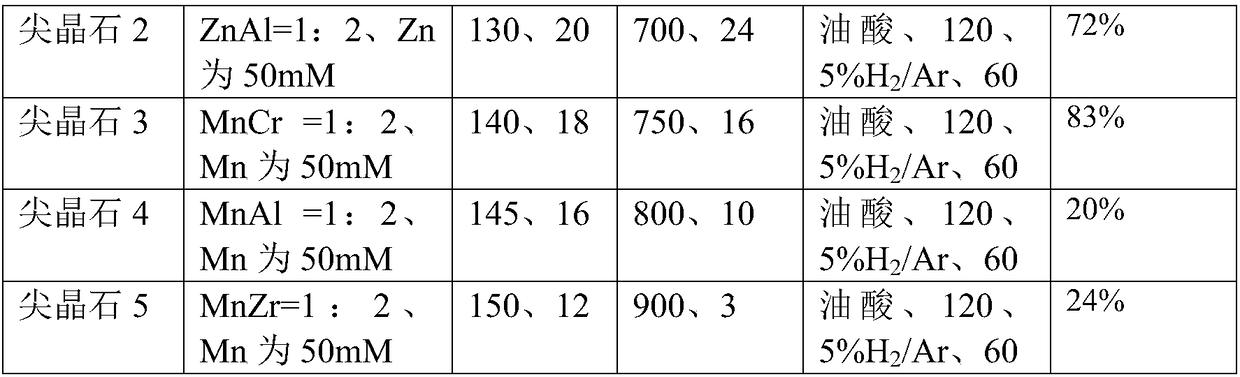
[0024] (1), the etching method synthesizes the ZnO material with polar surface:
[0025] (1) Weigh 4 parts, each part of 0.446g (1.5mmol) Zn(NO3) 2 6H2O in 4 containers, then weigh 0.300g (7.5mmol), 0.480g (12mmol), 0.720g ( 18mmol) and 1.200g (30mmol) NaOH were sequentially added to the above four containers, and then 30ml of deionized water was added to each of the four containers, and stirred for more than 0.5h to make the solution evenly mixed. The temperature was raised to 160°C, the reaction time was 20 hours, and the precipitate was decomposed into zinc oxide; naturally cooled to room temperature. The reaction solution was centrifuged to collect the precipitate after centrifugation, and washed twice with deionized water to obtain ZnO oxide;
[0026] Get wherein the product of 0.480g (12mmol) NaOH consumption carries out following processing:
[0027] (2) Use etchant such as oleic acid, urotropine, ethylenediamine, ammonia w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| mesopore | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com