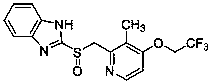
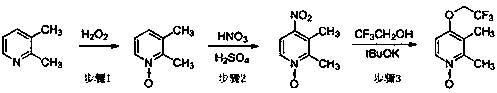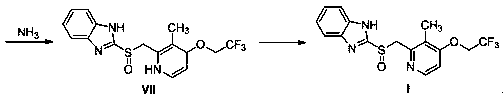Method for preparing lansoprazole
A technology for lansoprazole and benzimidazole, which is applied in the field of preparation of lansoprazole, can solve the problems of high discharge of three wastes, high environmental protection cost, long reaction steps and the like, and achieves high total yield and easy operation. , the effect of short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Preparation of trifluoroethoxyacrolein (IV)
[0042] Add 7.5 g (75 mmol) trifluoroethanol and 75 mL tetrahydrofuran to a 250 mL reaction flask, cool down to 0 °C, add 3.4 g (30 mmol) potassium tert-butoxide, add dropwise 4.1 g (75 mmol) propynaldehyde, After dropping, the temperature was controlled at 0°C for 15 minutes. After the reaction was complete, 140 mL of water was added, extracted three times with 200 mL of dichloromethane, the organic phases were combined, and concentrated under reduced pressure at 35°C to obtain trifluoroethoxyacrolein with a yield of 92.6% and a GC purity of 98.72%.
[0043] (2) Preparation of benzimidazole-2-sulfenyl chloride (V)
[0044] Add 10.0 g (67 mmol) of 2-mercaptobenzimidazole and 120 mL of chloroform to a 250 mL reaction flask, cool down to 0°C, add 11.9 g (100 mmol) of thionyl chloride dropwise, after dropping, control the temperature at 0°C for reaction 1 h. After the reaction was completed, it was concentrated under redu...
Embodiment 2
[0052] (1) Preparation of trifluoroethoxyacrolein (IV)
[0053] Add 7.5 g (75 mmol) trifluoroethanol and 75 mL tetrahydrofuran to a 250 mL reaction flask, cool down to 0°C, add 1.7 g (15 mmol) potassium tert-butoxide, add dropwise 3.8 g (71 mmol) propiolealdehyde, After dropping, the temperature was controlled at 0°C for 15 minutes. After the reaction was completed, 140 mL of water was added, extracted three times with 200 mL of dichloromethane, the organic phases were combined, dried with 10.0 g of anhydrous sodium sulfate, and concentrated under reduced pressure at 35°C to obtain trifluoroethoxyacrolein, yield: 87.4 %, GC purity: 98.12%.
[0054] (2) Preparation of benzimidazole-2-sulfenyl chloride (V)
[0055] Add 10.0 g (67 mmol) of 2-mercaptobenzimidazole and 120 mL of dichloromethane to a 250 mL reaction flask, cool down to 0°C, add 13.6 g (114 mmol) of thionyl chloride dropwise, after dropping, control the temperature at 0 ℃ for 1 h. After completion of the reaction...
Embodiment 3
[0063] (1) Preparation of trifluoroethoxyacrolein (IV)
[0064] Add 7.5 g (75 mmol) trifluoroethanol and 75 mL tetrahydrofuran to a 250 mL reaction flask, cool down to 0 °C, add 2.0 g (30 mmol) sodium ethoxide, add dropwise 4.1 g (75 mmol) propynaldehyde, dropwise , and the temperature was controlled at 25°C for 15 minutes. After the reaction was completed, 140 mL of water was added, extracted three times with 200 mL of dichloromethane, the organic phases were combined, dried with 10.0 g of anhydrous sodium sulfate, and concentrated under reduced pressure at 35°C to obtain trifluoroethoxyacrolein, yield: 92.6 %, GC purity: 98.50%.
[0065] (2) Preparation of benzimidazole-2-sulfenyl chloride (V)
[0066] Add 10.0 g (67 mmol) of 2-mercaptobenzimidazole and 120 mL of dichloromethane to a 250 mL reaction flask, cool down to 0°C, add 8.0 g (67 mmol) of thionyl chloride dropwise, after dropping, heat up to reflux React for 1 h. After completion of the reaction, concentrate unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com