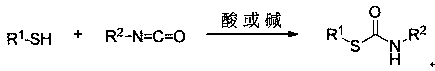Method of preparing sulfo-carbamate compound based on photocatalysis
A carbamate and compound technology, which is applied in the field of preparation of thiocarbamate compounds based on photocatalysis, can solve the problems of human injury, cumbersome operation, and high energy consumption, and avoid the use of metal reagents and reaction energy. Clean, process safe and reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] At room temperature, in a 15 mL reaction tube, add p-methylthiophenol (0.2 mmol), ethyl isocyanoacetate (0.5 mmol), photocatalyst Rose Bengal (0.004 mmol), ethyl acetate 0.5 mL and water 0.5 mL, mix well, and then under the irradiation of 3w blue LED light, stir and react in the air for 3h. After the completion of the reaction as detected by TLC, ethyl acetate was added for extraction 3 times, and the extract was concentrated under reduced pressure in a vacuum (0.08Mpa) to no solvent to obtain a crude product, which was then purified with petroleum ether and ethyl acetate at a volume ratio of 5:1. Washing with a mixed eluent, followed by flash column chromatography on a silica gel column, the thiocarbamate product of this example was obtained as 42.0 mg of a yellow oil, with a yield of 83%.
[0042] 1 H NMR (CDCl 3 , 500 MHz, ppm): δ 7.39 (d, J = 8.0 Hz, 2H), 7.16 (d, J =8.0 Hz, 2H), 5.82 (s, 1H), 4.11 (q, J = 7.2 Hz, 2H), 3.94 (d, J = 5.1 Hz,2H), ...
Embodiment 2
[0044]
[0045] At room temperature, add m-methylthiophenol (0.2 mmol), ethyl isocyanoacetate (0.5 mmol), photocatalyst water-soluble eosin (0.004 mmol), ethyl acetate 0.5 mL and water 0.5 mL in sequence in a 15 mL reaction tube , mixed evenly, and then stirred and reacted in the air for 3 hours under the irradiation of a 3w blue LED light. After the completion of the reaction as detected by TLC, ethyl acetate was added for extraction 3 times, and the extract was concentrated under reduced pressure in a vacuum (0.08Mpa) to no solvent to obtain a crude product, which was then purified with petroleum ether and ethyl acetate at a volume ratio of 5:1. Washing with a mixed eluent, followed by flash column chromatography on a silica gel column, the thiocarbamate product of this example was obtained as 38.4 mg of a yellow oil, with a yield of 76%.
[0046] 1 H NMR (CDCl 3 , 500 MHz, ppm): δ 7.41 (s, 1H), 7.39 (d, J = 7.9 Hz, 1H), 7.32 (d, J = 7.6 Hz, 1H), 7.25 (d, J = 7...
Embodiment 3
[0048]
[0049] At room temperature, add 23.6 μL (0.2 mmol) of o-methylthiophenol, 55 μL (0.5 mmol) of ethyl isocyanoacetate, photocatalyst Rose Bengal (0.004 mmol), 0.5 mL of isocyanide and 0.5 mL of water, mix well, and then under the irradiation of 3w blue LED light, stir and react in the air for 3h. After the completion of the reaction as detected by TLC, ethyl acetate was added for extraction 3 times, and the extract was concentrated under reduced pressure in a vacuum (0.08Mpa) to no solvent to obtain a crude product, which was then purified with petroleum ether and ethyl acetate at a volume ratio of 5:1. Washing with a mixed eluent, followed by flash column chromatography on a silica gel column, the thiocarbamate product of this example was obtained as 44.3 mg of a yellow oil, with a yield of 88%.
[0050] 1 H NMR (CDCl 3 , 500 MHz, ppm): δ 7.58 (d, J = 7.7 Hz, 1H), 7.38-7.34 (m,2H), 7.26-7.23 (m, 1H), 5.89 (s, 1H), 4.18 (q, J = 7.2 Hz, 2H), 4.00 (d, J =5.2 H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com