Diamine monomer containing asymmetric fluorophore structure as well as preparation method and application thereof
The technology of diamine monomer and fluorophore is applied in the field of diamine monomer containing asymmetric fluorophore structure and its preparation, and can solve the problems of difficulty in having electronically controlled fluorescence performance, weak triphenylamine fluorescence, high driving voltage and the like, Achieve enhanced solubility and solid-state fluorescence, improved contrast, and faster response times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The present invention also provides a preparation method of the diamine monomer containing an asymmetric fluorophore structure, comprising the following steps:
[0049] 4-fluoronitrobenzene, R 2 -NH 2 , triethylamine and dimethyl sulfoxide are mixed, nucleophilic substitution reaction I occurs, and a compound having the structure shown in formula II is obtained;
[0050] The compound having the structure shown in formula II, copper powder, potassium carbonate, 18-crown-6, R 1 -X is mixed with o-dichlorobenzene, and Ullman reaction occurs to obtain a compound with the structure shown in formula III; the R 1 X in -X is Cl, Br or I;
[0051] Mix the compound having the structure shown in formula III, Pd / C, hydrazine hydrate and dioxane, and undergo reduction reaction I to obtain the compound having the structure shown in formula IV;
[0052] Mix the compound having the structure shown in formula IV, 4-fluoronitrobenzene, cesium fluoride and dimethyl sulfoxide, and unde...
Embodiment 1
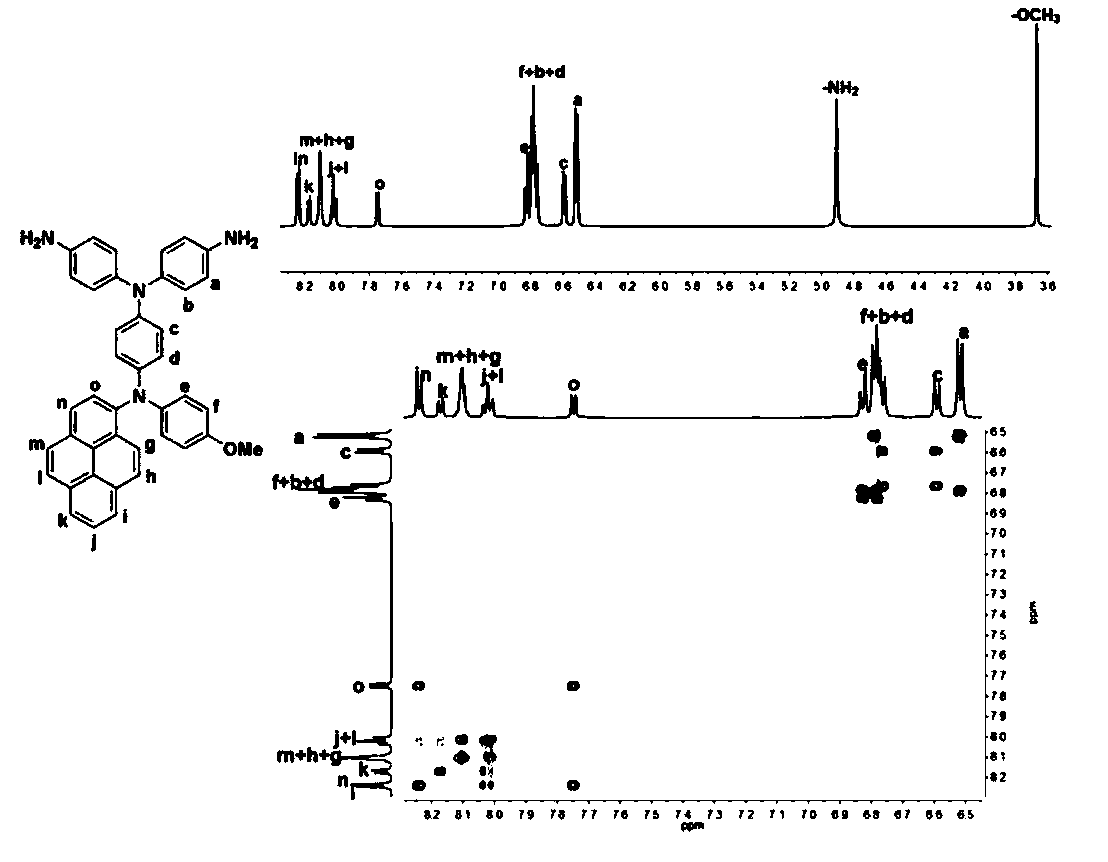
[0168] The preparation of N,N-bis(4-aminophenyl)-N'-4-methoxyphenyl-N'-1-aminopyrene-1,4-phenylenediamine, its structural formula is as follows:
[0169]
[0170] Under nitrogen, add 35.3g (250mmol) of 4-fluoronitrobenzene, 40.0g (325mmol) of p-methoxyaniline, 32.9g (325mmol) of triethyl amine, then added 273 mL of dimethyl sulfoxide, and reacted at 85°C for 36h. Discharge in ice-water mixture at room temperature, filter with suction, and recrystallize the filter cake with ethanol and N,N-dimethylformamide to obtain 4-nitro-4'-methoxydiphenylamine in orange-red needle-like crystals 45g, yield 74%;
[0171]Under nitrogen, add 10.0 g (35.6 mmol) of 1-bromopyrene, 9.2 g (37.4 mmol) of 4-nitro-4'- Methoxydiphenylamine, 9.0g (142.2mmol) of copper powder, 19.6g (142.2mmol) of potassium carbonate, 4.7g (17.8mmol) of 18-crown-6, then add 60ml of o-dichlorobenzene and react at 160°C 18h; Suction filtration while hot, and o-dichlorobenzene was distilled off under reduced pressure,...
Embodiment 2
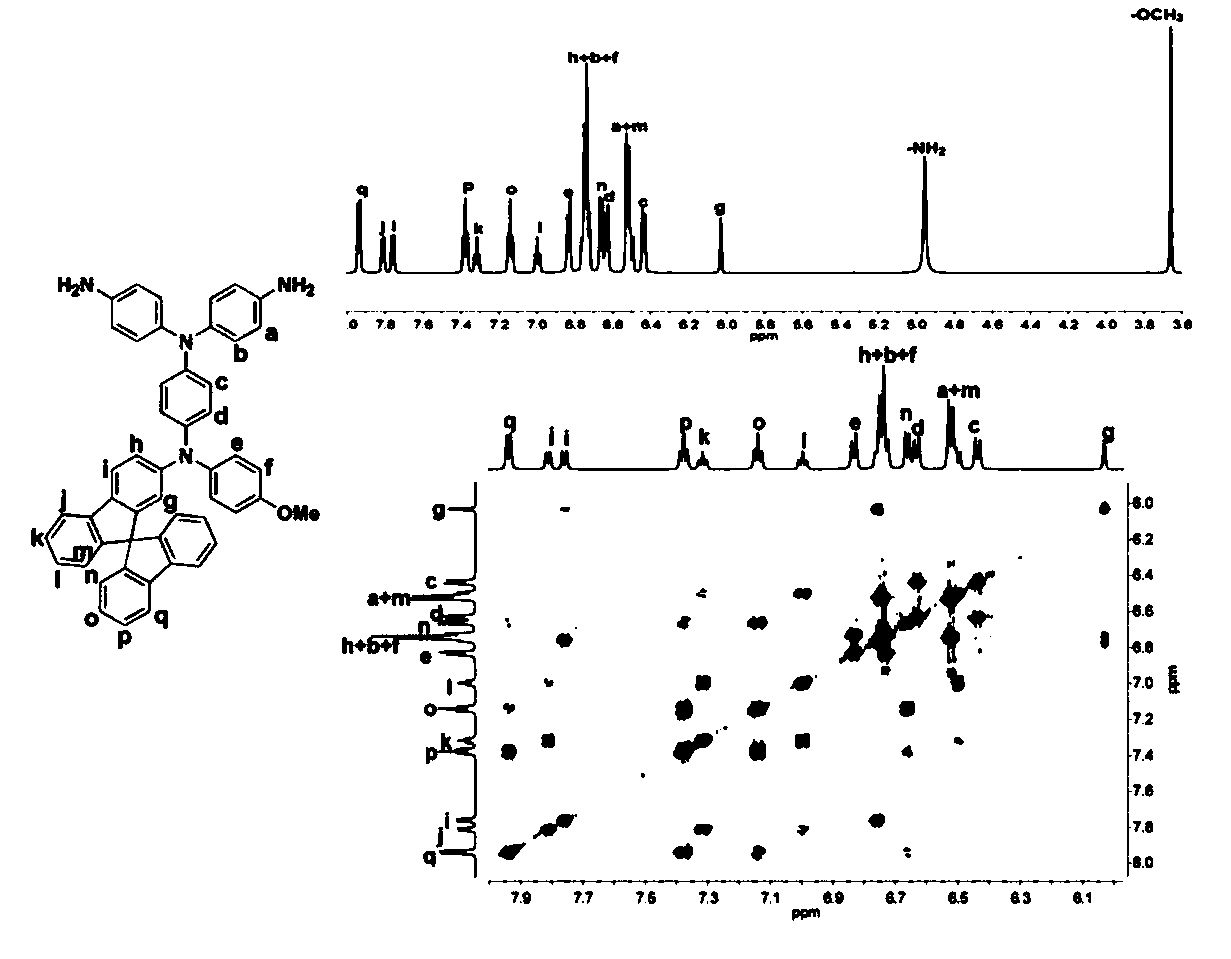
[0178] Preparation of N,N-bis(4-aminophenyl)-N'-4-methoxyphenyl-N'-2-amino-9,9'-spirobifluorene-1,4-phenylenediamine, The N,N-bis(4-aminophenyl)-N'-4-methoxyphenyl-N'-2-amino-9,9'-spirobifluorene-1,4-phenylenediamine The structural formula is:
[0179]
[0180] The preparation of 4-nitro-4'-methoxydiphenylamine is the same as in Example 1;
[0181] Under nitrogen, 9 g (22.8 mmol) of 2-bromo-9,9'-spirobifluorene, 6.2 g (25.2 mmol) of 4 -Nitro-4'-methoxydiphenylamine, 5.7g (91.2mmol) of copper powder, 12.6g (91.2mmol) of potassium carbonate, 3.0g (11.4mmol) of 18-crown-6, and then add 48mL of Dichlorobenzene, reacted at 160°C for 18 hours; suction filtered while it was hot, distilled o-dichlorobenzene under reduced pressure, and recrystallized the obtained crude product with ethanol and N,N-dimethylacetamide (V:V=1:1), Obtained 9.9 g of 4-nitrophenyl-4'-methoxyphenyl-2-amino-9,9'-spirobifluorene with a yield of 78%;
[0182] Add 9.0 g (16.2 mmol) of 4-nitrophenyl-4'-metho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com