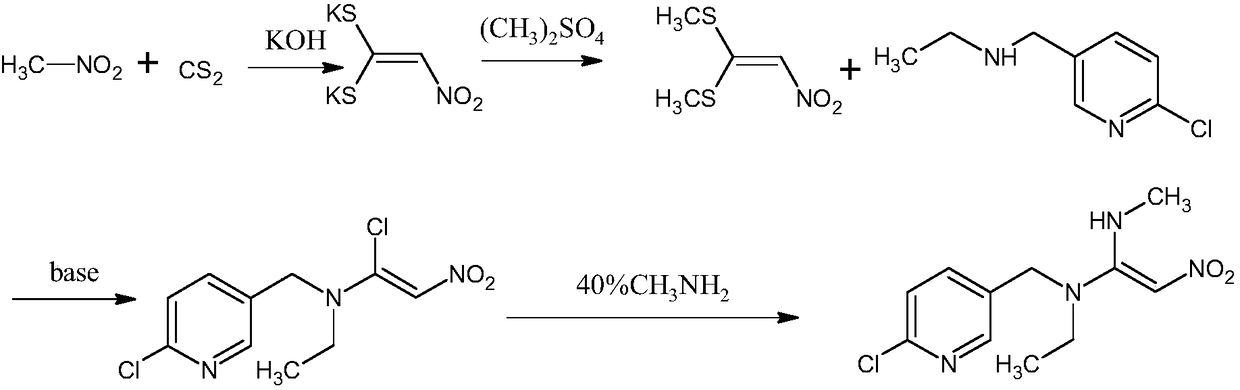
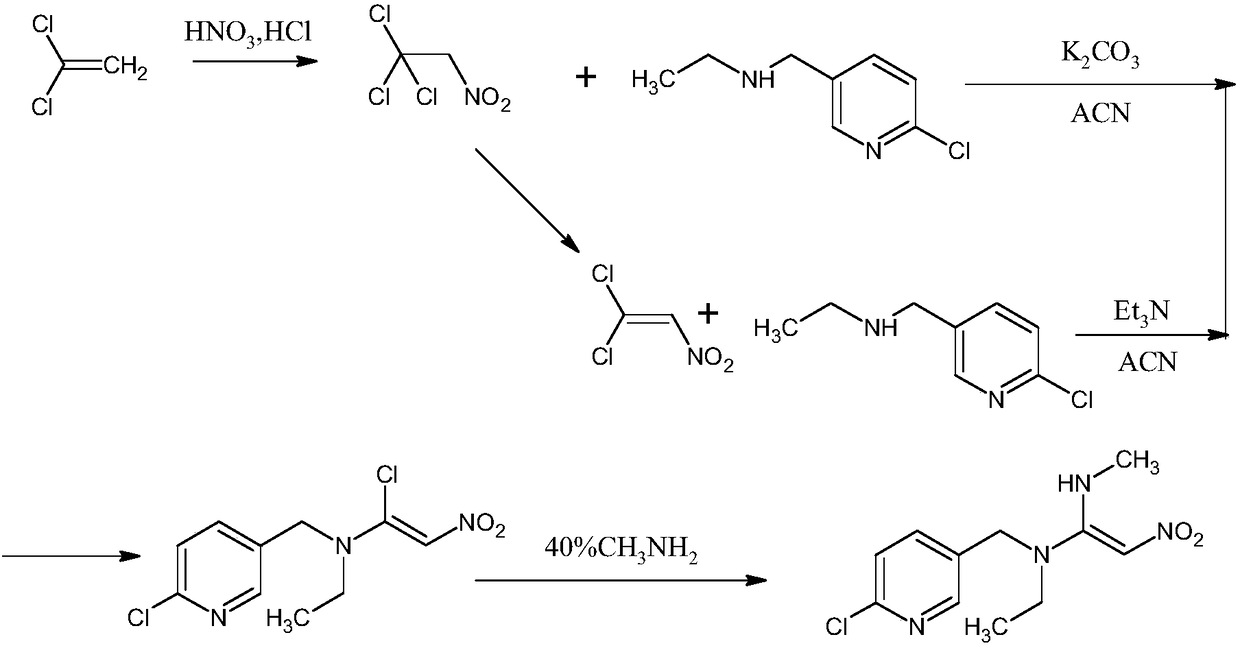
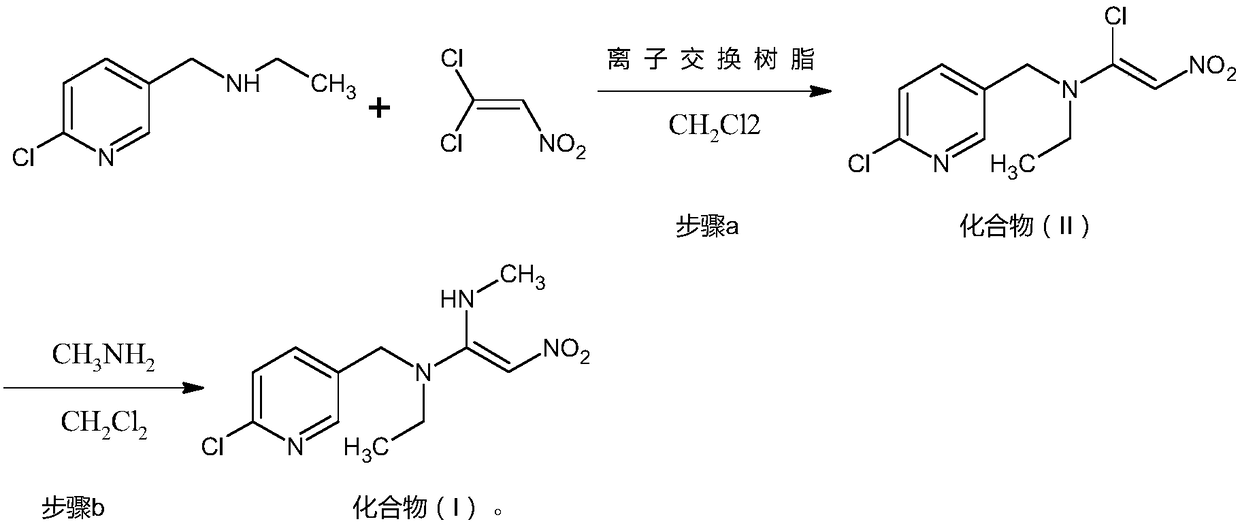
Preparation method of nitenpyram
A technology of nitenpyram and monomethylamine, which is applied in the field of preparation of nitenpyram, can solve the problems of high environmental protection pressure, difficult recovery, low reaction yield, etc., and achieves mild reaction conditions, green process, and high reaction yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 nitenpyram compound (I)
[0030] Put 1200kg of dichloromethane into a 2000L reactor, add 171kg (1kmol) of N-ethyl-2-chloro-5-pyridinemethanamine under stirring, 60L ion exchange resin 201x7, cool to internal temperature -10°C, drop 1 , A solution of 157kg (1.1kmol) of 1-dichloro-2-nitroethylene and 320kg of dichloromethane was added dropwise in 3 hours, and the internal temperature was controlled below 0°C. After dropping, keep warm at 0°C for 3 hours. After the reaction is complete, a solution containing compound (II) is obtained. Continue to lower the temperature of this solution to the internal temperature of -10°C, slowly pass in 46.5kg (1.5kmol) of monomethylamine gas, control the internal temperature below 0°C, and feed the calculated amount of monomethylamine gas for 3 hours, then keep warm at 0°C for reaction 3 hours. Complete, filter, wash the ion exchange resin with dichloromethane, concentrate the filtrate to dryness, add 28...
Embodiment 2
[0031] The preparation of embodiment 2 nitenpyram compound (I)
[0032] Put 1200kg of chloroform into the 2000L reaction kettle, throw in 171kg (1kmol) of N-ethyl-2-chloro-5-pyridinemethanamine under stirring, 70L ion exchange resin D201, cool to the inner temperature of 0°C, and drop 1,1- The solution of 142kg (1kmol) of dichloro-2-nitroethylene and 320kg of chloroform was added dropwise in 3 hours, and the internal temperature was controlled below 10°C. After dropping, the temperature was raised and kept at 20° C. for 3 hours. After the reaction is complete, a solution containing compound (II) is obtained. Continue to lower the temperature of the solution to an internal temperature of 0°C, slowly pass in 31kg (1kmol) of monomethylamine gas, control the internal temperature below 10°C, and feed the calculated amount of monomethylamine gas for 3 hours, then keep the temperature at 20°C for 3 hours. Complete, filter, dichloromethane washes the ion exchange resin, concentrates...
Embodiment 3
[0033] The preparation of embodiment 3 nitenpyram compound (I)
[0034] Put 1200kg of dichloroethane into the 2000L reactor, add 171kg (1koml) of N-ethyl-2-chloro-5-pyridinemethanamine under stirring, 60L ion exchange resin D301, cool to the inner temperature of 20°C, drop 1 , A solution of 170kg (1.2koml) of 1-dichloro-2-nitroethylene and 320kg of dichloroethane was added dropwise in 3 hours, and the internal temperature was controlled below 25°C. After dropping, the temperature was raised to 40°C and the reaction was kept for 2 hours. After the reaction is complete, a solution containing compound (II) is obtained. Continue to lower the temperature of this solution to an internal temperature of 20°C, slowly drop in 155kg (2kmol) of 40% monomethylamine solution, control the internal temperature below 25°C, finish the dripping in 3 hours, then raise the temperature to 40°C and keep it warm for 2 hours. Complete, filter, wash the ion exchange resin with dichloromethane, separa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com